Pancreatic ductal cells in development, regeneration, and neoplasia
- PMID: 22133881
- PMCID: PMC3225990
- DOI: 10.1172/JCI57131
Pancreatic ductal cells in development, regeneration, and neoplasia
Abstract
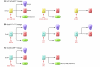
The pancreas is a complex organ comprised of three critical cell lineages: islet (endocrine), acinar, and ductal. This review will focus upon recent insights and advances in the biology of pancreatic ductal cells. In particular, emphasis will be placed upon the regulation of ductal cells by specific transcriptional factors during development as well as the underpinnings of acinar-ductal metaplasia as an important adaptive response during injury and regeneration. We also address the potential contributions of ductal cells to neoplastic transformation, specifically in pancreatic ductal adenocarcinoma.
Figures


References
-
- Cano DA, Hebrok M, Zenker M. Pancreatic development and disease. Gastroenterology. 2007;132(2):745–762. - PubMed

