Expansion of CD8+ T cells lacking Sema4D/CD100 during HIV-1 infection identifies a subset of T cells with decreased functional capacity
- PMID: 22134167
- PMCID: PMC3265199
- DOI: 10.1182/blood-2010-12-324848
Expansion of CD8+ T cells lacking Sema4D/CD100 during HIV-1 infection identifies a subset of T cells with decreased functional capacity
Abstract
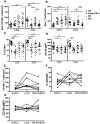
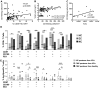
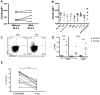
Sema4D, also known as CD100, is a constitutively expressed immune semaphorin on T cells and NK cells. CD100 has important immune regulatory functions that improve antigen-specific priming by antigen-presenting cells, and can also act as a costimulatory molecule on T cells. We investigated the consequence of HIV-1 infection on CD100 expression by T cells, and whether CD100 expression signifies functionally competent effector cells. CD100 expression on T cells from healthy individuals was compared with HIV-1-infected subjects including elite controllers, noncontrollers, and patients receiving antiretroviral therapy. The frequency and fluorescence intensity of CD100 on CD8(+) and CD4(+) T cells were decreased during HIV-1 infection. Furthermore, the absolute number of CD100-expressing CD8(+) T cells was positively associated with the magnitude of HIV-1-specific T-cell responses. CD8(+) T cells lacking CD100 expression were functionally impaired and present in increased numbers in HIV-1-infected individuals. The number of CD100(-)CD8(+) T cells positively correlated with T-cell immunosenescence, immune activation, and viral load. Loss of CD100 expression appears to result from direct antigen stimulation, as in vitro cytokine exposure and viral replication did not significantly impact CD100 expression. These data suggest that loss of CD100 expression probably plays an important role in dysfunctional immunity in HIV-1 infection.
Figures



References
-
- Elhabazi A, Delaire S, Bensussan A, Boumsell L, Bismuth G. Biological activity of soluble CD100. I. The extracellular region of CD100 is released from the surface of T lymphocytes by regulated proteolysis. J Immunol. 2001;166(7):4341–4347. - PubMed
-
- Tamagnone L, Artigiani S, Chen H, et al. Plexins are a large family of receptors for transmembrane, secreted, and GPI-anchored semaphorins in vertebrates. Cell. 1999;99(1):71–80. - PubMed
-
- Kumanogoh A, Watanabe C, Lee I, et al. Identification of CD72 as a lymphocyte receptor for the class IV semaphorin CD100: a novel mechanism for regulating B cell signaling. Immunity. 2000;13(5):621–631. - PubMed
-
- Kumanogoh A, Suzuki K, Ch'ng E, et al. Requirement for the lymphocyte semaphorin, CD100, in the induction of antigen-specific T cells and the maturation of dendritic cells. J Immunol. 2002;169(3):1175–1181. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI68498/AI/NIAID NIH HHS/United States
- AI 76 174/AI/NIAID NIH HHS/United States
- T32 HL007185/HL/NHLBI NIH HHS/United States
- K24AI069994/AI/NIAID NIH HHS/United States
- K24 AI069994/AI/NIAID NIH HHS/United States
- UL1 RR024131/RR/NCRR NIH HHS/United States
- R01 AI087145/AI/NIAID NIH HHS/United States
- P30 AI027763/AI/NIAID NIH HHS/United States
- U01 AI034993/AI/NIAID NIH HHS/United States
- R01 AI068498/AI/NIAID NIH HHS/United States
- P30 AI27763/AI/NIAID NIH HHS/United States
- U01-AI-034993/AI/NIAID NIH HHS/United States
- T32 AI060530/AI/NIAID NIH HHS/United States
- R24 AI067039/AI/NIAID NIH HHS/United States
- 5T32HL007185/HL/NHLBI NIH HHS/United States
- P30AI27763/AI/NIAID NIH HHS/United States
- 2T32AI060530-06/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

