AAV-mediated gene transfer in the perinatal period results in expression of FVII at levels that protect against fatal spontaneous hemorrhage
- PMID: 22134170
- PMCID: PMC3271720
- DOI: 10.1182/blood-2011-09-377630
AAV-mediated gene transfer in the perinatal period results in expression of FVII at levels that protect against fatal spontaneous hemorrhage
Abstract
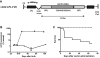
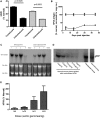
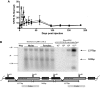
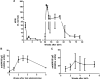
We explored adeno-associated viral vector (AAV)-mediated gene transfer in the perinatal period in animal models of severe congenital factor VII (FVII) deficiency, a disease associated with early postnatal life-threatening hemorrhage. In young adult mice with plasma FVII < 1% of normal, a single tail vein administration of AAV (1 × 10(13) vector genomes [vg]/kg) resulted in expression of murine FVII at 266% ± 34% of normal for ≥ 67 days, which mediated protection against fatal hemorrhage and significantly improved survival. Codon optimization of human FVII (hFVIIcoop) improved AAV transgene expression by 37-fold compared with the wild-type hFVII cDNA. In adult macaques, a single peripheral vein injection of 2 × 10(11) vg/kg of the hFVIIcoop AAV vector resulted in therapeutic levels of hFVII expression that were equivalent in males (10.7% ± 3.1%) and females (12.3% ± 0.8%). In utero delivery of this vector in the third trimester to fetal monkeys conferred expression of hFVII at birth of 20.4% ± 3.7%, with a gradual decline to > 1% by 7 weeks. Re-administration of an alternative serotype at 12 months postnatal age increased hFVII levels to 165% ± 6.2% of normal, which remained at therapeutic levels for a further 28 weeks without toxicity. Thus, perinatal AAV-mediated gene transfer shows promise for disorders with onset of pathology early after birth.
Figures

Similar articles
-
Sustained correction of FVII deficiency in dogs using AAV-mediated expression of zymogen FVII.Blood. 2016 Feb 4;127(5):565-71. doi: 10.1182/blood-2015-09-671420. Epub 2015 Dec 23. Blood. 2016. PMID: 26702064 Free PMC article.
-
Management of pregnancy in women with factor VII deficiency: A case series.Haemophilia. 2020 Jul;26(4):652-656. doi: 10.1111/hae.14086. Epub 2020 Jun 26. Haemophilia. 2020. PMID: 32590881
-
Enhancing transduction of the liver by adeno-associated viral vectors.Gene Ther. 2009 Jan;16(1):60-9. doi: 10.1038/gt.2008.137. Epub 2008 Aug 14. Gene Ther. 2009. PMID: 18701909 Free PMC article.
-
AAV-mediated gene transfer for hemophilia.Ann N Y Acad Sci. 2001 Dec;953:64-74. doi: 10.1111/j.1749-6632.2001.tb11361.x. Ann N Y Acad Sci. 2001. PMID: 11795424 Review.
-
Congenital factor VII deficiency: therapy with recombinant activated factor VII -- a critical appraisal.Haemophilia. 2006 Jan;12(1):19-27. doi: 10.1111/j.1365-2516.2006.01180.x. Haemophilia. 2006. PMID: 16409171 Review.
Cited by
-
AAV-mediated BMP7 gene therapy counteracts insulin resistance and obesity.Mol Ther Methods Clin Dev. 2022 Mar 16;25:190-204. doi: 10.1016/j.omtm.2022.03.007. eCollection 2022 Jun 9. Mol Ther Methods Clin Dev. 2022. PMID: 35434177 Free PMC article.
-
High-efficiency transduction of spinal cord motor neurons by intrauterine delivery of integration-deficient lentiviral vectors.J Control Release. 2018 Mar 10;273:99-107. doi: 10.1016/j.jconrel.2017.12.029. Epub 2017 Dec 28. J Control Release. 2018. PMID: 29289570 Free PMC article.
-
De novo variations of ANK1 gene caused hereditary spherocytosis in two Chinese children by affecting pre-mRNA splicing.BMC Pediatr. 2023 Jan 16;23(1):23. doi: 10.1186/s12887-022-03795-0. BMC Pediatr. 2023. PMID: 36647015 Free PMC article.
-
In vivo lentiviral vector gene therapy to cure hereditary tyrosinemia type 1 and prevent development of precancerous and cancerous lesions.Nat Commun. 2022 Aug 25;13(1):5012. doi: 10.1038/s41467-022-32576-7. Nat Commun. 2022. PMID: 36008405 Free PMC article.
-
Development of operational immunologic tolerance with neonatal gene transfer in nonhuman primates: preliminary studies.Gene Ther. 2015 Nov;22(11):923-30. doi: 10.1038/gt.2015.65. Epub 2015 Aug 23. Gene Ther. 2015. PMID: 26333349 Free PMC article.
References
-
- Nathwani AC, Tuddenham EG. Epidemiology of coagulation disorders. Baillieres Clin Haematol. 1992;5(2):383–439. - PubMed
-
- Pehlivanov B, Milchev N, Kroumov G. Factor VII deficiency and its treatment in delivery with recombinant factor VII. Eur J Obstet Gynecol Reprod Biol. 2004;116(2):237–238. - PubMed
-
- Mariani G, Konkle BA, Ingerslev J. Congenital factor VII deficiency: therapy with recombinant activated factor VII–a critical appraisal. Haemophilia. 2006;12(1):19–27. - PubMed
-
- Levi D, Pefkarou A, Fort JA, DeFaria W, Tzakis AG. Liver transplantation for factor VII deficiency. Transplantation. 2001;72(11):1836–1837. - PubMed
-
- Lapecorella M, Mariani G. Factor VII deficiency: defining the clinical picture and optimizing therapeutic options. Haemophilia. 2008;14(6):1170–1175. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

