Pro-oxidant and antioxidant effects of N-acetylcysteine regulate doxorubicin-induced NF-kappa B activity in leukemic cells
- PMID: 22134636
- PMCID: PMC3337722
- DOI: 10.1039/c1mb05315a
Pro-oxidant and antioxidant effects of N-acetylcysteine regulate doxorubicin-induced NF-kappa B activity in leukemic cells
Abstract
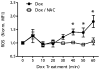
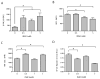
Clinical debate has arisen over the consequences of antioxidant supplementation during cancer chemotherapy. While antioxidants may impede the efficacy of chemotherapy by scavenging reactive oxygen species and free radicals, it is also possible that antioxidants alleviate unwanted chemotherapy-induced toxicity, thus allowing for increased chemotherapy doses. These contradictory assertions suggest that antioxidant supplementation during chemotherapy treatment can have varied outcomes depending on the cellular context. To gain a more robust understanding of the role that antioxidants play in chemotherapy, we investigated the dose-dependent effects of the antioxidant, N-acetylcysteine (NAC), on the redox-mediated regulation of intracellular signaling. In this study, we systematically evaluated the effect of Dox-induced ROS on the NF-κB pathway in a pediatric acute lymphoblastic leukemia (ALL) cell line by measuring the thiol-based oxidative modifications of redox-sensitive proteins within the pathway. We report a functional consequence of NAC supplementation during doxorubicin (Dox) chemotherapy administration via the NF-kappa B (NF-κB) signal transduction pathway. The ability of NAC to alter Dox-induced NF-κB activity is contingent on the ROS-mediated S-glutathionylation of IKK-β. Moreover, the NAC-dependent alteration of intracellular glutathione redox balance, through pro-oxidant and antioxidant mechanisms, can be exploited to either promote or inhibit Dox-induced NF-κB activity in an NAC-concentration-dependent manner. We developed an electron-transfer-based computational model that predicts the effect of NAC pretreatment on Dox-induced NF-κB signaling for a range of NAC and Dox treatment combinations.
Figures




Similar articles
-
Suppression of human prostate cancer PC-3 cell growth by N-acetylcysteine involves over-expression of Cyr61.Toxicol In Vitro. 2011 Feb;25(1):199-205. doi: 10.1016/j.tiv.2010.10.020. Epub 2010 Nov 3. Toxicol In Vitro. 2011. PMID: 21055460
-
Morphine inhibits doxorubicin-induced reactive oxygen species generation and nuclear factor kappaB transcriptional activation in neuroblastoma SH-SY5Y cells.Biochem J. 2007 Sep 1;406(2):215-21. doi: 10.1042/BJ20070186. Biochem J. 2007. PMID: 17542780 Free PMC article.
-
N-acetylcysteine alleviates pulmonary alveolar proteinosis induced by indium-tin oxide nanoparticles in male rats: involvement of the NF-κB signaling pathway.Ecotoxicol Environ Saf. 2022 Aug;241:113812. doi: 10.1016/j.ecoenv.2022.113812. Epub 2022 Jun 29. Ecotoxicol Environ Saf. 2022. PMID: 36068741
-
Molecular mechanisms of N-acetylcysteine actions.Cell Mol Life Sci. 2003 Jan;60(1):6-20. doi: 10.1007/s000180300001. Cell Mol Life Sci. 2003. PMID: 12613655 Free PMC article. Review.
-
[The application of N-acetylcysteine in optimization of specific pharmacological therapies].Pol Merkur Lekarski. 2017 Sep 29;43(255):140-144. Pol Merkur Lekarski. 2017. PMID: 28987048 Review. Polish.
Cited by
-
Redox-Active Mn Porphyrin-based Potent SOD Mimic, MnTnBuOE-2-PyP(5+), Enhances Carbenoxolone-Mediated TRAIL-Induced Apoptosis in Glioblastoma Multiforme.Stem Cell Rev Rep. 2016 Feb;12(1):140-55. doi: 10.1007/s12015-015-9628-2. Stem Cell Rev Rep. 2016. PMID: 26454429 Free PMC article.
-
Second-generation proteasome inhibitor carfilzomib enhances doxorubicin-induced cytotoxicity and apoptosis in breast cancer cells.Oncotarget. 2016 Nov 8;7(45):73697-73710. doi: 10.18632/oncotarget.12048. Oncotarget. 2016. PMID: 27655642 Free PMC article.
-
Non-Lethal Effects of N-Acetylcysteine on Xylella fastidiosa Strain De Donno Biofilm Formation and Detachment.Microorganisms. 2019 Dec 5;7(12):656. doi: 10.3390/microorganisms7120656. Microorganisms. 2019. PMID: 31817370 Free PMC article.
-
Modeling the Pro-inflammatory Tumor Microenvironment in Acute Lymphoblastic Leukemia Predicts a Breakdown of Hematopoietic-Mesenchymal Communication Networks.Front Physiol. 2016 Aug 19;7:349. doi: 10.3389/fphys.2016.00349. eCollection 2016. Front Physiol. 2016. PMID: 27594840 Free PMC article.
-
N-acetyl-cysteine blunts 6-hydroxydopamine- and L-buthionine-sulfoximine-induced apoptosis in human mesenchymal stromal cells.Mol Biol Rep. 2019 Aug;46(4):4423-4435. doi: 10.1007/s11033-019-04897-2. Epub 2019 May 30. Mol Biol Rep. 2019. PMID: 31147858
References
-
- Gupta D, Lis CG, Birdsall TC, Grutsch JF. Supportive Care Cancer. 2005;13:912–919. - PubMed
-
- Richardson MA, Sanders T, Palmer JL, Greisinger A, Singletary SE. J Clin Oncol. 2000;18:2505–2514. - PubMed
-
- Labriola D, Livingston R. Oncology (Williston Park) 1999;13:1003–1008. discussion 1008, 1011-1002. - PubMed
-
- Chinery R, Brockman JA, Peeler MO, Shyr Y, Beauchamp RD, Coffey RJ. Nat Med. 1997;3:1233–1241. - PubMed
-
- Mediavilla MD, Cos S, Sanchez-Barcelo EJ. Life Sci. 1999;65:415–420. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

