Ammonia-oxidizing archaea have more important role than ammonia-oxidizing bacteria in ammonia oxidation of strongly acidic soils
- PMID: 22134644
- PMCID: PMC3329103
- DOI: 10.1038/ismej.2011.168
Ammonia-oxidizing archaea have more important role than ammonia-oxidizing bacteria in ammonia oxidation of strongly acidic soils
Abstract
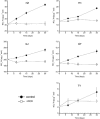
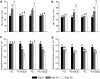
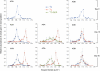
Increasing evidence demonstrated the involvement of ammonia-oxidizing archaea (AOA) in the global nitrogen cycle, but the relative contributions of AOA and ammonia-oxidizing bacteria (AOB) to ammonia oxidation are still in debate. Previous studies suggest that AOA would be more adapted to ammonia-limited oligotrophic conditions, which seems to be favored by protonation of ammonia, turning into ammonium in low-pH environments. Here, we investigated the autotrophic nitrification activity of AOA and AOB in five strongly acidic soils (pH<4.50) during microcosm incubation for 30 days. Significantly positive correlations between nitrate concentration and amoA gene abundance of AOA, but not of AOB, were observed during the active nitrification. (13)CO(2)-DNA-stable isotope probing results showed significant assimilation of (13)C-labeled carbon source into the amoA gene of AOA, but not of AOB, in one of the selected soil samples. High levels of thaumarchaeal amoA gene abundance were observed during the active nitrification, coupled with increasing intensity of two denaturing gradient gel electrophoresis bands for specific thaumarchaeal community. Addition of the nitrification inhibitor dicyandiamide (DCD) completely inhibited the nitrification activity and CO(2) fixation by AOA, accompanied by decreasing thaumarchaeal amoA gene abundance. Bacterial amoA gene abundance decreased in all microcosms irrespective of DCD addition, and mostly showed no correlation with nitrate concentrations. Phylogenetic analysis of thaumarchaeal amoA gene and 16S rRNA gene revealed active (13)CO(2)-labeled AOA belonged to groups 1.1a-associated and 1.1b. Taken together, these results provided strong evidence that AOA have a more important role than AOB in autotrophic ammonia oxidation in strongly acidic soils.
Figures





Similar articles
-
Improving Nitrogen Use Efficiency in Aerobic Rice Based on Insights Into the Ecophysiology of Archaeal and Bacterial Ammonia Oxidizers.Front Plant Sci. 2022 Jun 13;13:913204. doi: 10.3389/fpls.2022.913204. eCollection 2022. Front Plant Sci. 2022. PMID: 35769304 Free PMC article. Review.
-
[Nitrification Activity and Autotrophic Nitrifiers in Long-term Fertilized Acidic Upland Soils].Huan Jing Ke Xue. 2017 Aug 8;38(8):3473-3482. doi: 10.13227/j.hjkx.201701064. Huan Jing Ke Xue. 2017. PMID: 29964959 Chinese.
-
Differential contributions of ammonia oxidizers and nitrite oxidizers to nitrification in four paddy soils.ISME J. 2015 May;9(5):1062-75. doi: 10.1038/ismej.2014.194. Epub 2014 Oct 10. ISME J. 2015. PMID: 25303715 Free PMC article.
-
Effects of Different Land Use Types on Active Autotrophic Ammonia and Nitrite Oxidizers in Cinnamon Soils.Appl Environ Microbiol. 2021 May 26;87(12):e0009221. doi: 10.1128/AEM.00092-21. Epub 2021 May 26. Appl Environ Microbiol. 2021. PMID: 33837020 Free PMC article.
-
Diversity, physiology, and niche differentiation of ammonia-oxidizing archaea.Appl Environ Microbiol. 2012 Nov;78(21):7501-10. doi: 10.1128/AEM.01960-12. Epub 2012 Aug 24. Appl Environ Microbiol. 2012. PMID: 22923400 Free PMC article. Review.
Cited by
-
Bacterial and Archaeal Diversities in Maotai Section of the Chishui River, China.Curr Microbiol. 2016 Dec;73(6):924-929. doi: 10.1007/s00284-016-1142-5. Epub 2016 Sep 27. Curr Microbiol. 2016. PMID: 27671292
-
Effects of Land-Use Type and Flooding on the Soil Microbial Community and Functional Genes in Reservoir Riparian Zones.Microb Ecol. 2022 Feb;83(2):393-407. doi: 10.1007/s00248-021-01746-3. Epub 2021 Apr 23. Microb Ecol. 2022. PMID: 33893533
-
Relationships Between Soil Microbial Diversities Across an Aridity Gradient in Temperate Grasslands : Soil Microbial Diversity Relationships.Microb Ecol. 2023 Apr;85(3):1013-1027. doi: 10.1007/s00248-022-01997-8. Epub 2022 Apr 2. Microb Ecol. 2023. PMID: 35364696
-
Improving Nitrogen Use Efficiency in Aerobic Rice Based on Insights Into the Ecophysiology of Archaeal and Bacterial Ammonia Oxidizers.Front Plant Sci. 2022 Jun 13;13:913204. doi: 10.3389/fpls.2022.913204. eCollection 2022. Front Plant Sci. 2022. PMID: 35769304 Free PMC article. Review.
-
Spatial distribution patterns of ammonia-oxidizing archaea abundance in subtropical forests at early and late successional stages.Sci Rep. 2015 Nov 13;5:16587. doi: 10.1038/srep16587. Sci Rep. 2015. PMID: 26565069 Free PMC article.
References
-
- Amberger A. Research on dicyandiamide as a nitrification inhibitor and future outlook. Commun Soil Sci Plant Anal. 1989;20:1933–1955.
-
- Berg IA, Kockelkorn D, Buckel W, Fuchs G. A 3-hydroxypropionate/4-hydroxybutyrate autotrophic carbon dioxide assimilation pathway in archaea. Science. 2007;318:1782–1786. - PubMed
-
- Brochier-Armanet C, Boussau B, Gribaldo S, Forterre P. Mesophilic crenarchaeota: proposal for a third archaeal phylum, the thaumarchaeota. Nat Rev Microbiol. 2008;6:245–252. - PubMed
-
- De Boer W, Kowalchuk GA. Nitrification in acid soils: micro-organisms and mechanisms. Soil Biol Biochem. 2001;33:853–866.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

