Evaluation of oral chemotherapy with capecitabine and cyclophosphamide plus thalidomide and prednisone in prostate cancer patients
- PMID: 22134838
- PMCID: PMC11824262
- DOI: 10.1007/s00432-011-1101-2
Evaluation of oral chemotherapy with capecitabine and cyclophosphamide plus thalidomide and prednisone in prostate cancer patients
Abstract
Purpose: The aim of this study was to evaluate clinical outcomes of second-line chemotherapy with capecitabine and cyclophosphamide (CTX) plus thalidomide and prednisone in refractory advanced castrate-resistant prostate cancer (CRPC) patients.
Methods: We retrospectively reviewed patients with advanced CRPC who had previously progressed to first-line docetaxel-based chemotherapy. Patients were given second-line chemotherapy with capecitabine and CTX plus thalidomide and prednisone throughout the course. Patients were evaluated for response and toxicity, and treatment was continued until the disease progression or excessive toxicity was noted.
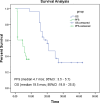
Results: From April 2007 to February 2010, a total of 28 patients (median age, 72.8 ± 2.9 years) received second-line chemotherapy. The median cycle and duration of metronomic chemotherapy were six (range: 1-12) cycles and 6.3 (range 1.5-20.5) months, respectively. Prostatic-specific antigen was decreased by more than 50% in 10 (35.7%) of the 28 patients. All patients had bone metastases, and 8 patients (28.6%) had measurable soft tissue lesions. Among the 8 patients, 1 patient achieved partial response, and 3 patients had stabilized disease. With a median follow-up time of 29.5 (95% CI, 26.4-33.4) months, median composite progression-free survival and overall survival were 4.7 (95% CI, 3.4-5.7) months and 19.5 (95% CI, 18.9-25.5) months, respectively. No grade 3-4 toxicity was observed, and none of the patients experienced grade 3-4 hematological and nonhematological toxicities.
Conclusions: These data suggested that oral combination second-line chemotherapy with capecitabine and CTX plus thalidomide and prednisone offers promising activity with an excellent safety profile for patients with advanced CRPC.
Figures

Similar articles
-
Comparison of first-line chemotherapy including escalated BEACOPP versus chemotherapy including ABVD for people with early unfavourable or advanced stage Hodgkin lymphoma.Cochrane Database Syst Rev. 2017 May 25;5(5):CD007941. doi: 10.1002/14651858.CD007941.pub3. Cochrane Database Syst Rev. 2017. PMID: 28541603 Free PMC article.
-
Gemcitabine-based chemotherapy for advanced biliary tract carcinomas.Cochrane Database Syst Rev. 2018 Apr 6;4(4):CD011746. doi: 10.1002/14651858.CD011746.pub2. Cochrane Database Syst Rev. 2018. PMID: 29624208 Free PMC article.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
Phase II study of capecitabine plus oxaliplatin (XELOX) as first-line treatment and followed by maintenance of capecitabine in patients with metastatic colorectal cancer.J Cancer Res Clin Oncol. 2010 Apr;136(4):503-10. doi: 10.1007/s00432-009-0682-5. Epub 2009 Sep 24. J Cancer Res Clin Oncol. 2010. PMID: 19777259 Free PMC article. Clinical Trial.
-
Efficacy and safety of capecitabine in combination with docetaxel and mitomycin C in patients with pre-treated pancreatic, gallbladder, and bile duct carcinoma.J Cancer Res Clin Oncol. 2010 Dec;136(12):1845-51. doi: 10.1007/s00432-010-0843-6. Epub 2010 Mar 12. J Cancer Res Clin Oncol. 2010. PMID: 20224968 Free PMC article. Clinical Trial.
Cited by
-
Metronomic cyclophosphamide therapy in hormone-naive patients with non-metastatic biochemical recurrent prostate cancer: a phase II trial.Med Oncol. 2016 Aug;33(8):89. doi: 10.1007/s12032-016-0806-0. Epub 2016 Jul 11. Med Oncol. 2016. PMID: 27400698 Clinical Trial.
-
Metronomics: towards personalized chemotherapy?Nat Rev Clin Oncol. 2014 Jul;11(7):413-31. doi: 10.1038/nrclinonc.2014.89. Epub 2014 Jun 10. Nat Rev Clin Oncol. 2014. PMID: 24913374 Review.
-
Metronomic Chemotherapy: A Systematic Review of the Literature and Clinical Experience.J Oncol. 2019 Mar 20;2019:5483791. doi: 10.1155/2019/5483791. eCollection 2019. J Oncol. 2019. PMID: 31015835 Free PMC article. Review.
-
Clinical, pharmacodynamic and pharmacokinetic results of a prospective phase II study on oral metronomic vinorelbine and dexamethasone in castration-resistant prostate cancer patients.Invest New Drugs. 2016 Dec;34(6):760-770. doi: 10.1007/s10637-016-0385-0. Epub 2016 Aug 24. Invest New Drugs. 2016. PMID: 27557783 Clinical Trial.
-
External validity of the Tokuhashi score in patients with vertebral metastasis.J Cancer Res Clin Oncol. 2012 Sep;138(9):1493-500. doi: 10.1007/s00432-012-1222-2. Epub 2012 Apr 22. J Cancer Res Clin Oncol. 2012. PMID: 22526160 Free PMC article.
References
-
- Beer TM, Ryan CW, Venner PM, Petrylak DP, Chatta GS, Ruether JD, Redfern CH, Fehrenbacher L, Saleh MN, Waterhouse DM, Carducci MA, Vicario D, Dreicer R, Higano CS, Ahmann FR, Chi KN, Henner WD, Arroyo A, Clow FW; ASCENT Investigators (2007) Double-blinded randomized study of high-dose calcitriol plus docetaxel compared with placebo plus docetaxel in androgen-independent prostate cancer: a report from the ASCENT Investigators. J Clin Oncol 5(6):669–674 - PubMed
-
- Bertolini F, Paul S, Mancuso P, Monestiroli S, Gobbi A, Shaked Y, Kerbel RS (2003) Maximum tolerable dose and low-dose metronomic chemotherapy have opposite effects on the mobilization and viability of circulating endothelial progenitor cells. Cancer Res 63(15):4342–4346 - PubMed
-
- Bocci G, Nicolaou KC, Kerbel RS (2002) Protracted low-dose effects on human endothelial cell proliferation and survival in vitro reveal a selective antiangiogenic window for various chemotherapeutic drugs. Cancer Res 62(23):6938–6943 - PubMed
-
- Bocci G, Tuccori M, Emmenegger U, Liguori V, Falcone A, Kerbel RS, Del Tacca M (2005) Cyclophosphamide- methotrexate ‘metronomic’ chemotherapy for the palliative treatment of metastatic breast cancer.A comparative pharmacoeconomic evaluation. Ann Oncol 16(8):1243–1252 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

