Involvement of glomerular renin-angiotensin system (RAS) activation in the development and progression of glomerular injury
- PMID: 22134870
- PMCID: PMC3328682
- DOI: 10.1007/s10157-011-0568-0
Involvement of glomerular renin-angiotensin system (RAS) activation in the development and progression of glomerular injury
Abstract
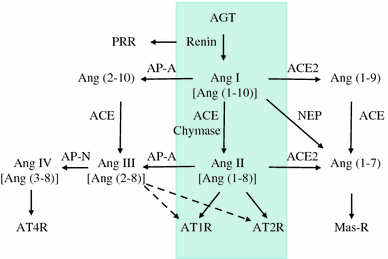
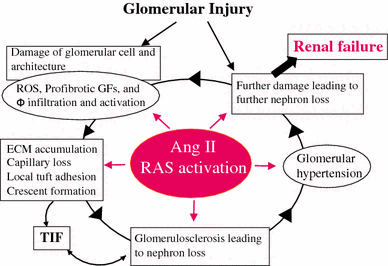
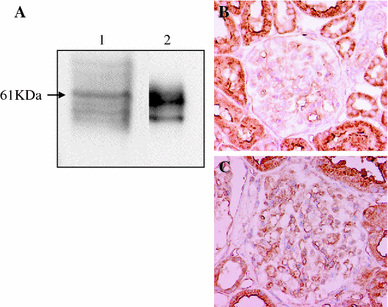
Recently, there has been a paradigm shift away from an emphasis on the role of the endocrine (circulating) renin-angiotensin system (RAS) in the regulation of the sodium and extracellular fluid balance, blood pressure, and the pathophysiology of hypertensive organ damage toward a focus on the role of tissue RAS found in many organs, including kidney. A tissue RAS implies that RAS components necessary for the production of angiotensin II (Ang II) reside within the tissue and its production is regulated within the tissue, independent of the circulating RAS. Locally produced Ang II plays a role in many physiological and pathophysiological processes such as hypertension, inflammation, oxidative stress, and tissue fibrosis. Both glomerular and tubular compartments of the kidney have the characteristics of a tissue RAS. The purpose of this article is to review the recent advances in tissue RAS research with a particular focus on the role of the glomerular RAS in the progression of renal disease.
Figures

References
-
- Tigerstedt R, Bergman PG. Niere und Kreislauf. Skand Arch Physiol. 1898;8:223–271.
-
- Dzau VJ. Tissue angiotensin and pathobiology of vascular disease: a unifying hypothesis. Hypertension. 2001;37:1047–1052. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

