Dentin phosphoprotein (DPP) activates integrin-mediated anchorage-dependent signals in undifferentiated mesenchymal cells
- PMID: 22134916
- PMCID: PMC3285302
- DOI: 10.1074/jbc.M111.290080
Dentin phosphoprotein (DPP) activates integrin-mediated anchorage-dependent signals in undifferentiated mesenchymal cells
Abstract
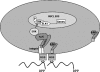
Dentin phosphoprotein (DPP), a major noncollagenous protein of the dentin matrix, is a highly acidic protein that binds Ca(2+) avidly and is thus linked to matrix mineralization. Here, we demonstrate that the RGD domain in DPP can bind to integrins on the cell surface of undifferentiated mesenchymal stem cells and pulp cells. This coupling generates intracellular signals that are channeled along cytoskeletal filaments and activate the non-receptor tyrosine kinase focal adhesion kinase, which plays a key role in signaling at sites of cellular adhesion. The putative focal adhesion kinase autophosphorylation site Tyr(397) is phosphorylated during focal adhesion assembly induced by DPP on the substrate. We further demonstrate that these intracellular signals propagate through the cytoplasm and activate anchorage-dependent ERK signaling. Activated ERK translocates to the nucleus and phosphorylates the transcription factor ELK-1, which in turn coordinates the expression of downstream target genes such as DMP1 and dentin sialoprotein (DSP). These studies suggest a novel paradigm demonstrating that extracellular DPP can induce intracellular signaling that can be propagated to the nucleus and thus alter gene activities.
Figures













References
-
- Feng J. Q., Luan X., Wallace J., Jing D., Ohshima T., Kulkarni A. B., D'Souza R. N., Kozak C. A., MacDougall M. (1998) Genomic organization, chromosomal mapping, and promoter analysis of the mouse dentin sialophosphoprotein (Dspp) gene, which codes for both dentin sialoprotein and dentin phosphoprotein. J. Biol. Chem. 273, 9457–9464 - PubMed
-
- George A., Srinivasan R., Thotakura S. R., Liu K., Veis A. (1999) Rat dentin matrix protein 3 is a compound protein of rat dentin sialoprotein and phosphophoryn. Connect. Tissue Res. 40, 49–57 - PubMed
-
- Yamakoshi Y., Hu J. C., Iwata T., Kobayashi K., Fukae M., Simmer J. P. (2006) Dentin sialophosphoprotein is processed by MMP-2 and MMP-20 in vitro and in vivo. J. Biol. Chem. 281, 38235–38243 - PubMed
-
- Yamakoshi Y., Hu J. C., Fukae M., Zhang H., Simmer J. P. (2005) Dentin glycoprotein: the protein in the middle of the dentin sialophosphoprotein chimera. J. Biol. Chem. 280, 17472–17479 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

