Novel Cβ-Cγ bond cleavages of tryptophan-containing peptide radical cations
- PMID: 22135037
- PMCID: PMC3264861
- DOI: 10.1007/s13361-011-0295-5
Novel Cβ-Cγ bond cleavages of tryptophan-containing peptide radical cations
Abstract
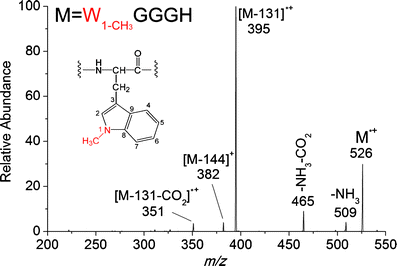
In this study, we observed unprecedented cleavages of the C(β)-C(γ) bonds of tryptophan residue side chains in a series of hydrogen-deficient tryptophan-containing peptide radical cations (M(•+)) during low-energy collision-induced dissociation (CID). We used CID experiments and theoretical density functional theory (DFT) calculations to study the mechanism of this bond cleavage, which forms [M - 116](+) ions. The formation of an α-carbon radical intermediate at the tryptophan residue for the subsequent C(β)-C(γ) bond cleavage is analogous to that occurring at leucine residues, producing the same product ions; this hypothesis was supported by the identical product ion spectra of [LGGGH - 43](+) and [WGGGH - 116](+), obtained from the CID of [LGGGH](•+) and [WGGGH](•+), respectively. Elimination of the neutral 116-Da radical requires inevitable dehydrogenation of the indole nitrogen atom, leaving the radical centered formally on the indole nitrogen atom ([Ind](•)-2), in agreement with the CID data for [WGGGH](•+) and [W(1-CH3)GGGH](•+); replacing the tryptophan residue with a 1-methyltryptophan residue results in a change of the base peak from that arising from a neutral radical loss (116 Da) to that arising from a molecule loss (131 Da), both originating from C(β)-C(γ) bond cleavage. Hydrogen atom transfer or proton transfer to the γ-carbon atom of the tryptophan residue weakens the C(β)-C(γ) bond and, therefore, decreases the dissociation energy barrier dramatically.
© The Author(s) 2011. This article is published with open access at Springerlink.com
Figures

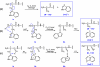
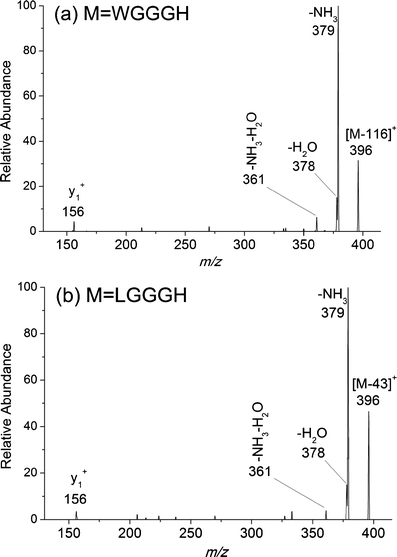

Similar articles
-
Mechanistic examination of Cβ-Cγ bond cleavages of tryptophan residues during dissociations of molecular peptide radical cations.J Phys Chem A. 2013 Feb 14;117(6):1059-68. doi: 10.1021/jp303562e. Epub 2012 Jul 18. J Phys Chem A. 2013. PMID: 22697598
-
Hydrogen atom transfer in the radical cations of tryptophan-containing peptides AW and WA studied by mass spectrometry, infrared multiple-photon dissociation spectroscopy, and theoretical calculations.Eur J Mass Spectrom (Chichester). 2019 Feb;25(1):112-121. doi: 10.1177/1469066718802547. Epub 2018 Oct 3. Eur J Mass Spectrom (Chichester). 2019. PMID: 30282467
-
Arginine-facilitated isomerization: radical-induced dissociation of aliphatic radical cationic glycylarginyl(iso)leucine tripeptides.J Phys Chem B. 2012 Jul 5;116(26):7627-34. doi: 10.1021/jp301882p. Epub 2012 Jun 20. J Phys Chem B. 2012. PMID: 22671034
-
Collision-induced fragmentations of the (M-H)- parent anions of underivatized peptides: an aid to structure determination and some unusual negative ion cleavages.Mass Spectrom Rev. 2002 Mar-Apr;21(2):87-107. doi: 10.1002/mas.10022. Mass Spectrom Rev. 2002. PMID: 12373746 Review.
-
Formation of peptide radical ions through dissociative electron transfer in ternary metal-ligand-peptide complexes.Eur J Mass Spectrom (Chichester). 2011;17(6):543-56. doi: 10.1255/ejms.1156. Eur J Mass Spectrom (Chichester). 2011. PMID: 22274945 Review.
Cited by
-
Structure and reactivity of the distonic and aromatic radical cations of tryptophan.J Am Soc Mass Spectrom. 2013 Apr;24(4):513-23. doi: 10.1007/s13361-013-0594-0. Epub 2013 Mar 20. J Am Soc Mass Spectrom. 2013. PMID: 23512424
-
Leveraging Electron Transfer Dissociation for Site Selective Radical Generation: Applications for Peptide Epimer Analysis.J Am Soc Mass Spectrom. 2017 Jul;28(7):1365-1373. doi: 10.1007/s13361-017-1627-x. Epub 2017 Apr 3. J Am Soc Mass Spectrom. 2017. PMID: 28374314 Free PMC article.
-
Radical additions to aromatic residues in peptides facilitate unexpected side chain and backbone losses.J Am Soc Mass Spectrom. 2014 Apr;25(4):626-35. doi: 10.1007/s13361-013-0810-y. Epub 2014 Feb 1. J Am Soc Mass Spectrom. 2014. PMID: 24488753
-
Fragmentation chemistry of [Met-Gly]•+, [Gly-Met]•+, and [Met-Met]•+ radical cations.J Am Soc Mass Spectrom. 2013 Apr;24(4):543-53. doi: 10.1007/s13361-013-0581-5. Epub 2013 Feb 26. J Am Soc Mass Spectrom. 2013. PMID: 23440718
-
Mechanistic investigation of phosphate ester bond cleavages of glycylphosphoserinyltryptophan radical cations under low-energy collision-induced dissociation.J Am Soc Mass Spectrom. 2013 Apr;24(4):554-62. doi: 10.1007/s13361-013-0597-x. Epub 2013 Mar 21. J Am Soc Mass Spectrom. 2013. PMID: 23516067
References
-
- Stults, J.T.; Arnott, D.: In: Burlingame, A.L. (ed.) Academic Press, Methods Enzymol 402, 245–289 (2005) - PubMed
-
- Jones JL, Dongré AR, Somogyi Á, Wysocki VH. Sequence Dependence of Peptide Fragmentation Efficiency Curves Determined by Electrospray Ionization/Surface-Induced Dissociation Mass Spectrometry. J. Am. Chem. Soc. 1994;116:8368–8369. doi: 10.1021/ja00097a055. - DOI
-
- Dongré AR, Jones JL, Somogyi Á, Wysocki VH. Influence of Peptide Composition, Gas-Phase Basicity, and Chemical Modification on Fragmentation Efficiency: Evidence for the Mobile Proton Model. J. Am. Chem. Soc. 1996;118:8365–8374. doi: 10.1021/ja9542193. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

