FXR protects lung from lipopolysaccharide-induced acute injury
- PMID: 22135065
- PMCID: PMC3248324
- DOI: 10.1210/me.2011-0042
FXR protects lung from lipopolysaccharide-induced acute injury
Abstract
Acute lung injury and its more severe form, acute respiratory distress syndrome, are characterized by an acute inflammatory response in the airspaces and lung parenchyma. The nuclear receptor farnesoid X receptor (FXR) is expressed in pulmonary artery endothelial cells. Here, we report a protective role of FXR in a lipopolysaccharide-induced mouse model of acute lung injury. Upon intratracheal injection of lipopolysaccharide, FXR-/- mice showed higher lung endothelial permeability, released more bronchoalveolar lavage cells to the alveoli, and developed acute pneumonia. Cell adhesion molecules were expressed at higher levels in FXR-/- mice as compared with control mice. Furthermore, lung regeneration was much slower in FXR-/- mice. In vitro experiments showed that FXR activation blocked TNFα-induced expression of P-selectin but stimulated proliferation of lung microvascular endothelial cells through up-regulation of Foxm1b. In addition, expression of a constitutively active FXR repressed the expression of proinflammatory genes and improved lung permeability and lung regeneration in FXR-/- mice. This study demonstrates a critical role of FXR in suppressing the inflammatory response in lung and promoting lung repair after injury.
Figures
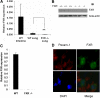
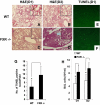



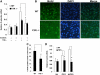
Comment in
-
Editorial: molecular endocrinology articles in the spotlight for January 2012.Mol Endocrinol. 2012 Jan;26(1):1. doi: 10.1210/me.2011-1345. Mol Endocrinol. 2012. PMID: 22210759 Free PMC article. No abstract available.
Similar articles
-
Atrial natriuretic peptide inhibits lipopolysaccharide-induced acute lung injury.Pulm Pharmacol Ther. 2014 Oct;29(1):24-30. doi: 10.1016/j.pupt.2014.01.003. Epub 2014 Jan 22. Pulm Pharmacol Ther. 2014. PMID: 24462877
-
Parkin regulates lipopolysaccharide-induced proinflammatory responses in acute lung injury.Transl Res. 2017 Mar;181:71-82. doi: 10.1016/j.trsl.2016.09.002. Epub 2016 Sep 13. Transl Res. 2017. PMID: 27693468
-
Farnesoid X receptor alleviates age-related proliferation defects in regenerating mouse livers by activating forkhead box m1b transcription.Hepatology. 2010 Mar;51(3):953-62. doi: 10.1002/hep.23390. Hepatology. 2010. PMID: 19998409 Free PMC article.
-
LRP1-Dependent BMPER Signaling Regulates Lipopolysaccharide-Induced Vascular Inflammation.Arterioscler Thromb Vasc Biol. 2017 Aug;37(8):1524-1535. doi: 10.1161/ATVBAHA.117.309521. Epub 2017 Jun 8. Arterioscler Thromb Vasc Biol. 2017. PMID: 28596374 Free PMC article.
-
Danger-associated molecular patterns (DAMPs) in acute lung injury.J Pathol. 2013 Jan;229(2):145-56. doi: 10.1002/path.4124. J Pathol. 2013. PMID: 23097158 Review.
Cited by
-
Farnesoid X receptor (FXR) as a potential therapeutic target for lung diseases: a narrative review.J Thorac Dis. 2024 Nov 30;16(11):8026-8038. doi: 10.21037/jtd-24-734. Epub 2024 Nov 29. J Thorac Dis. 2024. PMID: 39678849 Free PMC article. Review.
-
Role of farnesoid X receptor in inflammation and resolution.Inflamm Res. 2015 Jan;64(1):9-20. doi: 10.1007/s00011-014-0780-y. Epub 2014 Nov 7. Inflamm Res. 2015. PMID: 25376338 Review.
-
Enhanced Bilosomal Properties Resulted in Optimum Pharmacological Effects by Increased Acidification Pathways.Pharmaceutics. 2021 Jul 31;13(8):1184. doi: 10.3390/pharmaceutics13081184. Pharmaceutics. 2021. PMID: 34452145 Free PMC article.
-
Regulation of Lung Macrophage Activation and Oxidative Stress Following Ozone Exposure by Farnesoid X Receptor.Toxicol Sci. 2020 Oct 1;177(2):441-453. doi: 10.1093/toxsci/kfaa111. Toxicol Sci. 2020. PMID: 32984886 Free PMC article.
-
Regulation of DDAH1 as a Potential Therapeutic Target for Treating Cardiovascular Diseases.Evid Based Complement Alternat Med. 2013;2013:619207. doi: 10.1155/2013/619207. Epub 2013 Jun 26. Evid Based Complement Alternat Med. 2013. PMID: 23878601 Free PMC article.
References
-
- Ware LB, Matthay MA. 2000. The acute respiratory distress syndrome. N Engl J Med 342:1334–1349 - PubMed
-
- Kalinichenko VV, Gusarova GA, Tan Y, Wang IC, Major ML, Wang X, Yoder HM, Costa RH, Costal RH. 2003. Ubiquitous expression of the forkhead box M1B transgene accelerates proliferation of distinct pulmonary cell types following lung injury. J Biol Chem 278:37888–37894 - PubMed
-
- Huang W, Ma K, Zhang J, Qatanani M, Cuvillier J, Liu J, Dong B, Huang X, Moore DD. 2006. Nuclear receptor-dependent bile acid signaling is required for normal liver regeneration. Science 312:233–236 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

