The expression and role of hyperpolarization-activated and cyclic nucleotide-gated channels in endocrine anterior pituitary cells
- PMID: 22135067
- PMCID: PMC3248322
- DOI: 10.1210/me.2011-1207
The expression and role of hyperpolarization-activated and cyclic nucleotide-gated channels in endocrine anterior pituitary cells
Abstract
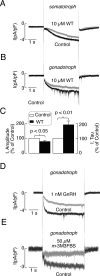
Pituitary cells fire action potentials independently of external stimuli, and such spontaneous electrical activity is modulated by a large variety of hypothalamic and intrapituitary agonists. Here, we focused on the potential role of hyperpolarization-activated and cyclic nucleotide-gated (HCN) channels in electrical activity of cultured rat anterior pituitary cells. Quantitative RT-PCR analysis showed higher level of expression of mRNA transcripts for HCN2 and HCN3 subunits and lower expression of HCN1 and HCN4 subunits in these cells. Western immunoblot analysis of lysates from normal and GH(3) immortalized pituitary cells showed bands with appropriate molecular weights for HCN2, HCN3, and HCN4. Electrophysiological experiments showed the presence of a slowly developing hyperpolarization-activated inward current, which was blocked by Cs(+) and ZD7288, in gonadotrophs, thyrotrophs, somatotrophs, and a fraction of lactotrophs, as well as in other unidentified pituitary cell types. Stimulation of adenylyl cyclase and addition of 8-Br-cAMP enhanced this current and depolarized the cell membrane, whereas 8-Br-cGMP did not alter the current and hyperpolarized the cell membrane. Both inhibition of basal adenylyl cyclase activity and stimulation of phospholipase C signaling pathway inhibited this current. Inhibition of HCN channels affected the frequency of firing but did not abolish spontaneous electrical activity. These experiments indicate that cAMP and cGMP have opposite effects on the excitability of endocrine pituitary cells, that basal cAMP production in cultured cells is sufficient to integrate the majority of HCN channels in electrical activity, and that depletion of phosphatidylinositol 4,5-bisphosphate caused by activation of phospholipase C silences them.
Figures





Similar articles
-
Dependence of hyperpolarisation-activated cyclic nucleotide-gated channel activity on basal cyclic adenosine monophosphate production in spontaneously firing GH3 cells.J Neuroendocrinol. 2006 Jul;18(7):484-93. doi: 10.1111/j.1365-2826.2006.01438.x. J Neuroendocrinol. 2006. PMID: 16774497
-
Involvement of hyperpolarization-activated, cyclic nucleotide-gated cation channels in dorsal root ganglion in neuropathic pain.Sheng Li Xue Bao. 2008 Oct 25;60(5):579-80. Sheng Li Xue Bao. 2008. PMID: 18958363
-
Functional expression of the human HCN3 channel.J Biol Chem. 2005 Oct 14;280(41):34635-43. doi: 10.1074/jbc.M502508200. Epub 2005 Jul 25. J Biol Chem. 2005. PMID: 16043489
-
Hyperpolarization-activated and cyclic nucleotide-gated channel proteins as emerging new targets in neuropathic pain.Rev Neurosci. 2019 Jul 26;30(6):639-649. doi: 10.1515/revneuro-2018-0094. Rev Neurosci. 2019. PMID: 30768426 Review.
-
Neurophysiology of HCN channels: from cellular functions to multiple regulations.Prog Neurobiol. 2014 Jan;112:1-23. doi: 10.1016/j.pneurobio.2013.10.001. Epub 2013 Oct 29. Prog Neurobiol. 2014. PMID: 24184323 Review.
Cited by
-
Ion Channels of Pituitary Gonadotrophs and Their Roles in Signaling and Secretion.Front Endocrinol (Lausanne). 2017 Jun 9;8:126. doi: 10.3389/fendo.2017.00126. eCollection 2017. Front Endocrinol (Lausanne). 2017. PMID: 28649232 Free PMC article. Review.
-
Inhibitory Effective Perturbations of Cilobradine (DK-AH269), A Blocker of HCN Channels, on the Amplitude and Gating of Both Hyperpolarization-Activated Cation and Delayed-Rectifier Potassium Currents.Int J Mol Sci. 2020 Mar 31;21(7):2416. doi: 10.3390/ijms21072416. Int J Mol Sci. 2020. PMID: 32244431 Free PMC article.
-
Constitutively active HCN channels constrain detrusor excitability and modulate evoked contractions of human bladder.Am J Clin Exp Urol. 2020 Oct 15;8(5):163-176. eCollection 2020. Am J Clin Exp Urol. 2020. PMID: 33235894 Free PMC article.
-
Characterization of Inhibitory Capability on Hyperpolarization-Activated Cation Current Caused by Lutein (β,ε-Carotene-3,3'-Diol), a Dietary Xanthophyll Carotenoid.Int J Mol Sci. 2022 Jun 28;23(13):7186. doi: 10.3390/ijms23137186. Int J Mol Sci. 2022. PMID: 35806190 Free PMC article.
-
Dependence of the excitability of pituitary cells on cyclic nucleotides.J Neuroendocrinol. 2012 Sep;24(9):1183-200. doi: 10.1111/j.1365-2826.2012.02335.x. J Neuroendocrinol. 2012. PMID: 22564128 Free PMC article. Review.
References
-
- Van Goor F, Zivadinovic D, Martinez-Fuentes AJ, Stojilkovic SS. 2001. Dependence of pituitary hormone secretion on the pattern of spontaneous voltage-gated calcium influx. Cell type-specific action potential secretion coupling. J Biol Chem 276:33840–33846 - PubMed
-
- Hille B. 2001. Ion channels of excitable cells. Sunderland, MA: Sinauer Associates, Inc
-
- Craven KB, Zagotta WN. 2006. CNG and HCN channels: two peas, one pod. Annu Rev Physiol 68:375–401 - PubMed
-
- Gauss R, Seifert R, Kaupp UB. 1998. Molecular identification of a hyperpolarization-activated channel in sea urchin sperm. Nature 393:583–587 - PubMed

