Suppressed prostate epithelial development with impaired branching morphogenesis in mice lacking stromal fibromuscular androgen receptor
- PMID: 22135068
- PMCID: PMC3248327
- DOI: 10.1210/me.2011-1189
Suppressed prostate epithelial development with impaired branching morphogenesis in mice lacking stromal fibromuscular androgen receptor
Erratum in
-
Corrigendum to: "Suppressed Prostate Epithelial Development with Impaired Branching Morphogenesis in Mice Lacking Stromal Fibromuscular Androgen Receptor".Endocrinology. 2020 Oct 1;161(10):bqaa148. doi: 10.1210/endocr/bqaa148. Endocrinology. 2020. PMID: 32966551 No abstract available.
Abstract
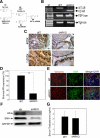
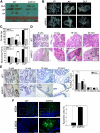
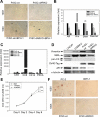
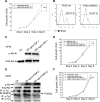
Using the cre-loxP system, we generated a new mouse model [double stromal androgen receptor knockout (dARKO)] with selectively deleted androgen receptor (AR) in both stromal fibroblasts and smooth muscle cells, and found the size of the anterior prostate (AP) lobes was significantly reduced as compared with those from wild-type littermate controls. The reduction in prostate size of the dARKO mouse was accompanied by impaired branching morphogenesis and partial loss of the infolding glandular structure. Further dissection found decreased proliferation and increased apoptosis of the prostate epithelium in the dARKO mouse AP. These phenotype changes were further confirmed with newly established immortalized prostate stromal cells (PrSC) from wild-type and dARKO mice. Mechanistically, IGF-1, placental growth factor, and secreted phosphoprotein-1 controlled by stromal AR were differentially expressed in PrSC-wt and PrSC-ARKO. Moreover, the conditioned media (CM) from PrSC-wt promoted prostate epithelium growth significantly as compared with CM from PrSC-dARKO. Finally, adding IGF-1/placental growth factor recombinant proteins into PrSC-dARKO CM was able to partially rescue epithelium growth. Together, our data concluded that stromal fibromuscular AR could modulate epithelium growth and maintain cellular homeostasis through identified growth factors.
Figures


Similar articles
-
Altered prostate epithelial development in mice lacking the androgen receptor in stromal fibroblasts.Prostate. 2012 Mar;72(4):437-49. doi: 10.1002/pros.21445. Epub 2011 Jul 7. Prostate. 2012. PMID: 21739465 Free PMC article.
-
Increased CK5/CK8-positive intermediate cells with stromal smooth muscle cell atrophy in the mice lacking prostate epithelial androgen receptor.PLoS One. 2011;6(7):e20202. doi: 10.1371/journal.pone.0020202. Epub 2011 Jul 6. PLoS One. 2011. PMID: 21754978 Free PMC article.
-
Altered prostate epithelial development and IGF-1 signal in mice lacking the androgen receptor in stromal smooth muscle cells.Prostate. 2011 Apr;71(5):517-24. doi: 10.1002/pros.21264. Epub 2010 Oct 13. Prostate. 2011. PMID: 20945497 Free PMC article.
-
Influence of stromal-epithelial interactions on androgen action.Endocr Relat Cancer. 2014 Aug;21(4):T147-60. doi: 10.1530/ERC-14-0138. Epub 2014 May 28. Endocr Relat Cancer. 2014. PMID: 24872510 Review.
-
Role of stromal-epithelial interactions in hormonal responses.Arch Histol Cytol. 2004 Dec;67(5):417-34. doi: 10.1679/aohc.67.417. Arch Histol Cytol. 2004. PMID: 15781983 Review.
Cited by
-
Loss of stromal androgen receptor leads to suppressed prostate tumourigenesis via modulation of pro-inflammatory cytokines/chemokines.EMBO Mol Med. 2012 Aug;4(8):791-807. doi: 10.1002/emmm.201101140. Epub 2012 Jun 29. EMBO Mol Med. 2012. PMID: 22745041 Free PMC article.
-
Polygenic risk score predicting susceptibility and outcome of benign prostatic hyperplasia in the Han Chinese.Hum Genomics. 2024 May 22;18(1):49. doi: 10.1186/s40246-024-00619-3. Hum Genomics. 2024. PMID: 38778357 Free PMC article.
-
Cellular Constituents of the Prostate Stroma: Key Contributors to Prostate Cancer Progression and Therapy Resistance.Cold Spring Harb Perspect Med. 2018 Aug 1;8(8):a030510. doi: 10.1101/cshperspect.a030510. Cold Spring Harb Perspect Med. 2018. PMID: 28490538 Free PMC article. Review.
-
Androgen receptor (AR) physiological roles in male and female reproductive systems: lessons learned from AR-knockout mice lacking AR in selective cells.Biol Reprod. 2013 Jul 25;89(1):21. doi: 10.1095/biolreprod.113.109132. Print 2013 Jul. Biol Reprod. 2013. PMID: 23782840 Free PMC article. Review.
-
The role of the androgen receptor in prostate development and benign prostatic hyperplasia: A review.Asian J Urol. 2020 Jul;7(3):191-202. doi: 10.1016/j.ajur.2019.10.003. Epub 2019 Oct 19. Asian J Urol. 2020. PMID: 32742923 Free PMC article. Review.
References
-
- Cunha GR, Chung LW, Shannon JM, Taguchi O, Fujii H. 1983. Hormone-induced morphogenesis and growth: role of mesenchymal-epithelial interactions. Recent Prog Horm Res 39:559–598 - PubMed
-
- Cunha GR, Chung LW. 1981. Stromal-epithelial interactions–I. Induction of prostatic phenotype in urothelium of testicular feminized (Tfm/y) mice. J Steroid Biochem 14:1317–1324 - PubMed
-
- Cunha GR, Cooke PS, Kurita T. 2004. Role of stromal-epithelial interactions in hormonal responses. Arch Histol Cytol 67:417–434 - PubMed
-
- Cunha GR, Ricke W, Thomson A, Marker PC, Risbridger G, Hayward SW, Wang YZ, Donjacour AA, Kurita T. 2004. Hormonal, cellular, and molecular regulation of normal and neoplastic prostatic development. J Steroid Biochem Mol Biol 92:221–236 - PubMed
-
- Thomson AA. 2008. Mesenchymal mechanisms in prostate organogenesis. Differentiation 76:587–598 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

