Moxifloxacin versus amoxicillin/clavulanic acid in outpatient acute exacerbations of COPD: MAESTRAL results
- PMID: 22135277
- PMCID: PMC3393767
- DOI: 10.1183/09031936.00090311
Moxifloxacin versus amoxicillin/clavulanic acid in outpatient acute exacerbations of COPD: MAESTRAL results
Erratum in
- Eur Respir J. 2012 Sep;40(3):800
Abstract
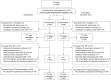
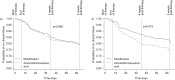
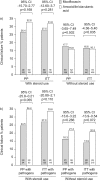
Bacterial infections causing acute exacerbations of chronic obstructive pulmonary disease (AECOPD) frequently require antibacterial treatment. More evidence is needed to guide antibiotic choice. The Moxifloxacin in Acute Exacerbations of Chronic Bronchitis TriaL (MAESTRAL) was a multiregional, randomised, double-blind non-inferiority outpatient study. Patients were aged ≥ 60 yrs, with an Anthonisen type I exacerbation, a forced expiratory volume in 1 s < 60% predicted and two or more exacerbations in the last year. Following stratification by steroid use patients received moxifloxacin 400 mg p.o. q.d. (5 days) or amoxicillin/clavulanic acid 875/125 mg p.o. b.i.d. (7 days). The primary end-point was clinical failure 8 weeks post-therapy in the per protocol population. Moxifloxacin was noninferior to amoxicillin/clavulanic acid at the primary end-point (111 (20.6%) out of 538, versus 114 (22.0%) out of 518, respectively; 95% CI -5.89-3.83%). In patients with confirmed bacterial AECOPD, moxifloxacin led to significantly lower clinical failure rates than amoxicillin/clavulanic acid (in the intent-to-treat with pathogens, 62 (19.0%) out of 327 versus 85 (25.4%) out of 335, respectively; p=0.016). Confirmed bacterial eradication at end of therapy was associated with higher clinical cure rates at 8 weeks post-therapy overall (p=0.0014) and for moxifloxacin (p=0.003). Patients treated with oral corticosteroids had more severe disease and higher failure rates. The MAESTRAL study showed that moxifloxacin was as effective as amoxicillin/clavulanic acid in the treatment of outpatients with AECOPD. Both therapies were well tolerated.
Conflict of interest statement
Statements of interest for all authors and the study itself can be found at
Figures
Comment in
-
Antibiotics in acute exacerbations of COPD: the good, the bad and the ugly.Eur Respir J. 2012 Jul;40(1):1-3. doi: 10.1183/09031936.00211911. Eur Respir J. 2012. PMID: 22753830 No abstract available.
Similar articles
-
A novel study design for antibiotic trials in acute exacerbations of COPD: MAESTRAL methodology.Int J Chron Obstruct Pulmon Dis. 2011;6:373-83. doi: 10.2147/COPD.S21071. Epub 2011 Jun 29. Int J Chron Obstruct Pulmon Dis. 2011. PMID: 21760724 Free PMC article. Clinical Trial.
-
Moxifloxacin monotherapy compared to amoxicillin-clavulanate plus roxithromycin for nonsevere community-acquired pneumonia in adults with risk factors.Eur J Clin Microbiol Infect Dis. 2005 Jun;24(6):367-76. doi: 10.1007/s10096-005-1347-1. Eur J Clin Microbiol Infect Dis. 2005. PMID: 15944847 Clinical Trial.
-
Randomized controlled trial of moxifloxacin compared with piperacillin-tazobactam and amoxicillin-clavulanate for the treatment of complicated intra-abdominal infections.Ann Surg. 2006 Aug;244(2):204-11. doi: 10.1097/01.sla.0000230024.84190.a8. Ann Surg. 2006. PMID: 16858182 Free PMC article. Clinical Trial.
-
Moxifloxacin in the management of exacerbations of chronic bronchitis and COPD.Int J Chron Obstruct Pulmon Dis. 2007;2(3):191-204. Int J Chron Obstruct Pulmon Dis. 2007. PMID: 18229559 Free PMC article. Review.
-
The use of moxifloxacin for acute exacerbations of chronic obstructive pulmonary disease and chronic bronchitis.Expert Rev Respir Med. 2012 Nov;6(5):481-92. doi: 10.1586/ers.12.50. Epub 2012 Oct 31. Expert Rev Respir Med. 2012. PMID: 23113693 Review.
Cited by
-
Prognostic factors for clinical failure of exacerbations in elderly outpatients with moderate-to-severe COPD.Int J Chron Obstruct Pulmon Dis. 2015 Jun 2;10:985-93. doi: 10.2147/COPD.S80926. eCollection 2015. Int J Chron Obstruct Pulmon Dis. 2015. PMID: 26082623 Free PMC article. Clinical Trial.
-
Effect of levofloxacin on neutrophilic airway inflammation in stable COPD: a randomized, double-blind, placebo-controlled trial.Int J Chron Obstruct Pulmon Dis. 2014 Feb 7;9:179-86. doi: 10.2147/COPD.S55419. eCollection 2014. Int J Chron Obstruct Pulmon Dis. 2014. PMID: 24532969 Free PMC article. Clinical Trial.
-
Treatment failure and hospital readmissions in severe COPD exacerbations treated with azithromycin versus placebo - a post-hoc analysis of the BACE randomized controlled trial.Respir Res. 2019 Oct 29;20(1):237. doi: 10.1186/s12931-019-1208-6. Respir Res. 2019. PMID: 31665017 Free PMC article. Clinical Trial.
-
Chronic Respiratory Infection in Patients with Chronic Obstructive Pulmonary Disease: What Is the Role of Antibiotics?Int J Mol Sci. 2017 Jun 23;18(7):1344. doi: 10.3390/ijms18071344. Int J Mol Sci. 2017. PMID: 28644389 Free PMC article. Review.
-
Comparison of the clinical characteristics and treatment outcomes of patients requiring hospital admission to treat eosinophilic and neutrophilic exacerbations of COPD.Int J Chron Obstruct Pulmon Dis. 2016 Oct 3;11:2467-2473. doi: 10.2147/COPD.S116072. eCollection 2016. Int J Chron Obstruct Pulmon Dis. 2016. PMID: 27757029 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
