Coexpression of ecto-5'-nucleotidase/CD73 with specific NTPDases differentially regulates adenosine formation in the rat liver
- PMID: 22135310
- PMCID: PMC3287391
- DOI: 10.1152/ajpgi.00165.2011
Coexpression of ecto-5'-nucleotidase/CD73 with specific NTPDases differentially regulates adenosine formation in the rat liver
Abstract
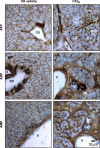
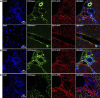
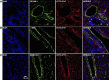
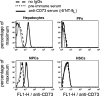
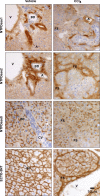
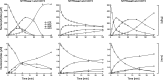
Ectonucleotidases modulate purinergic signaling by hydrolyzing ATP to adenosine. Here we characterized the impact of the cellular distribution of hepatic ectonucleotidases, namely nucleoside triphosphate diphosphohydrolase (NTPDase)1/CD39, NTPDase2/CD39L1, NTPDase8, and ecto-5'-nucleotidase/CD73, and of their specific biochemical properties, on the levels of P1 and P2 receptor agonists, with an emphasis on adenosine-producing CD73. Immunostaining and enzyme histochemistry showed that the distribution of CD73 (protein and AMPase activity) overlaps partially with those of NTPDase1, -2, and -8 (protein levels and ATPase and ADPase activities) in normal rat liver. CD73 is expressed in fibroblastic cells located underneath vascular endothelial cells and smooth muscle cells, which both express NTPDase1, in portal spaces in a distinct fibroblast population next to NTPDase2-positive portal fibroblasts, and in bile canaliculi, together with NTPDase8. In fibrotic rat livers, CD73 protein expression and activity are redistributed but still overlap with the NTPDases mentioned. The ability of the observed combinations of ectonucleotidases to generate adenosine over time was evaluated by reverse-phase HPLC with the recombinant rat enzymes at high "inflammatory" (500 μM) and low "physiological" (1 μM) ATP concentrations. Overall, ATP was rapidly converted to adenosine by the NTPDase1+CD73 combination, but not by the NTPDase2+CD73 combination. In the presence of NTPDase8 and CD73, ATP was sequentially dephosphorylated to the CD73 inhibitor ADP, and then to AMP, thus resulting in a delayed formation of adenosine. In conclusion, the specific cellular cocompartmentalization of CD73 with hepatic NTPDases is not redundant and may lead to the differential activation of P1 and P2 receptors, under normal and fibrotic conditions.
Figures



Similar articles
-
Cloning, purification, and identification of the liver canalicular ecto-ATPase as NTPDase8.Am J Physiol Gastrointest Liver Physiol. 2007 Mar;292(3):G785-95. doi: 10.1152/ajpgi.00293.2006. Epub 2006 Nov 9. Am J Physiol Gastrointest Liver Physiol. 2007. PMID: 17095758 Free PMC article.
-
Specificity of the ecto-ATPase inhibitor ARL 67156 on human and mouse ectonucleotidases.Br J Pharmacol. 2007 Sep;152(1):141-50. doi: 10.1038/sj.bjp.0707361. Epub 2007 Jul 2. Br J Pharmacol. 2007. PMID: 17603550 Free PMC article.
-
NTPDase and 5' ecto-nucleotidase expression profiles and the pattern of extracellular ATP metabolism in the Walker 256 tumor.Biochim Biophys Acta. 2007 Aug;1770(8):1259-65. doi: 10.1016/j.bbagen.2007.05.004. Epub 2007 May 21. Biochim Biophys Acta. 2007. PMID: 17574764
-
The role of the CD39-CD73-adenosine pathway in liver disease.J Cell Physiol. 2021 Feb;236(2):851-862. doi: 10.1002/jcp.29932. Epub 2020 Jul 10. J Cell Physiol. 2021. PMID: 32648591 Review.
-
Adenosine and adenosine receptors in colorectal cancer.Int Immunopharmacol. 2020 Oct;87:106853. doi: 10.1016/j.intimp.2020.106853. Epub 2020 Aug 2. Int Immunopharmacol. 2020. PMID: 32755765 Review.
Cited by
-
The Cholangiocyte Adenosine-IL-6 Axis Regulates Survival During Biliary Cirrhosis.Gene Expr. 2017 Nov 27;17(4):327-340. doi: 10.3727/105221617X15042723767876. Epub 2017 Sep 11. Gene Expr. 2017. PMID: 28893353 Free PMC article.
-
Activated hepatic stellate cells upregulate transcription of ecto-5'-nucleotidase/CD73 via specific SP1 and SMAD promoter elements.Am J Physiol Gastrointest Liver Physiol. 2012 Oct 15;303(8):G904-14. doi: 10.1152/ajpgi.00015.2012. Epub 2012 Aug 16. Am J Physiol Gastrointest Liver Physiol. 2012. PMID: 22899823 Free PMC article.
-
Purinergic signalling in liver diseases: Pathological functions and therapeutic opportunities.JHEP Rep. 2020 Jul 30;2(6):100165. doi: 10.1016/j.jhepr.2020.100165. eCollection 2020 Dec. JHEP Rep. 2020. PMID: 33103092 Free PMC article. Review.
-
Purinergic signalling in the liver in health and disease.Purinergic Signal. 2014 Mar;10(1):51-70. doi: 10.1007/s11302-013-9398-8. Epub 2013 Nov 24. Purinergic Signal. 2014. PMID: 24271096 Free PMC article. Review.
-
Deficits in endogenous adenosine formation by ecto-5'-nucleotidase/CD73 impair neuromuscular transmission and immune competence in experimental autoimmune myasthenia gravis.Mediators Inflamm. 2015;2015:460610. doi: 10.1155/2015/460610. Epub 2015 Jan 27. Mediators Inflamm. 2015. PMID: 25691808 Free PMC article.
References
-
- Airas L, Salmi M, Jalkanen S. Lymphocyte-vascular adhesion protein-2 is a novel 70-kDa molecule involved in lymphocyte adhesion to vascular endothelium. J Immunol 151: 4228–4238, 1993. - PubMed
-
- Bachmann S, Ramasubbu K. Immunohistochemical colocalization of the alpha-subunit of neutrophil NADPH oxidase and ecto-5′-nucleotidase in kidney and liver. Kidney Int 51: 479–482, 1997. - PubMed
-
- Baykov AA, Evtushenko OA, Avaeva SM. A malachite green procedure for orthophosphate determination and its use in alkaline phosphatase-based enzyme immunoassay. Anal Biochem 171: 266–270, 1988. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

