A TRPC5-regulated calcium signaling pathway controls dendrite patterning in the mammalian brain
- PMID: 22135323
- PMCID: PMC3248686
- DOI: 10.1101/gad.174060.111
A TRPC5-regulated calcium signaling pathway controls dendrite patterning in the mammalian brain
Abstract
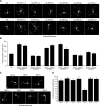
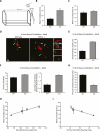
Transient receptor potential (TRP) channels have been implicated as sensors of diverse stimuli in mature neurons. However, developmental roles for TRP channels in the establishment of neuronal connectivity remain largely unexplored. Here, we identify an essential function for TRPC5, a member of the canonical TRP subfamily, in the regulation of dendrite patterning in the mammalian brain. Strikingly, TRPC5 knockout mice harbor long, highly branched granule neuron dendrites with impaired dendritic claw differentiation in the cerebellar cortex. In vivo RNAi analyses suggest that TRPC5 regulates dendrite morphogenesis in the cerebellar cortex in a cell-autonomous manner. Correlating with impaired dendrite patterning in the cerebellar cortex, behavioral analyses reveal that TRPC5 knockout mice have deficits in gait and motor coordination. Finally, we uncover the molecular basis of TRPC5's function in dendrite patterning. We identify the major protein kinase calcium/calmodulin-dependent kinase II β (CaMKIIβ) as a critical effector of TRPC5 function in neurons. Remarkably, TRPC5 forms a complex specifically with CaMKIIβ, but not the closely related kinase CaMKIIα, and thereby induces the CaMKIIβ-dependent phosphorylation of the ubiquitin ligase Cdc20-APC at the centrosome. Accordingly, centrosomal CaMKIIβ signaling mediates the ability of TRPC5 to regulate dendrite morphogenesis in neurons. Our findings define a novel function for TRPC5 that couples calcium signaling to a ubiquitin ligase pathway at the centrosome and thereby orchestrates dendrite patterning and connectivity in the brain.
© 2011 by Cold Spring Harbor Laboratory Press
Figures




Similar articles
-
A CaMKIIβ signaling pathway at the centrosome regulates dendrite patterning in the brain.Nat Neurosci. 2011 Jul 3;14(8):973-83. doi: 10.1038/nn.2857. Nat Neurosci. 2011. PMID: 21725312 Free PMC article.
-
A centrosomal Cdc20-APC pathway controls dendrite morphogenesis in postmitotic neurons.Cell. 2009 Jan 23;136(2):322-36. doi: 10.1016/j.cell.2008.11.050. Cell. 2009. PMID: 19167333 Free PMC article.
-
TRPC5 channel is the mediator of neurotrophin-3 in regulating dendritic growth via CaMKIIα in rat hippocampal neurons.J Neurosci. 2012 Jul 4;32(27):9383-95. doi: 10.1523/JNEUROSCI.6363-11.2012. J Neurosci. 2012. PMID: 22764246 Free PMC article.
-
Canonical transient receptor potential 5.Handb Exp Pharmacol. 2007;(179):109-23. doi: 10.1007/978-3-540-34891-7_6. Handb Exp Pharmacol. 2007. PMID: 17217053 Review.
-
TRPC4 and TRPC5: receptor-operated Ca2+-permeable nonselective cation channels.Cell Calcium. 2003 May-Jun;33(5-6):441-50. doi: 10.1016/s0143-4160(03)00055-1. Cell Calcium. 2003. PMID: 12765689 Review.
Cited by
-
The odyssey of the TR(i)P journey to the cellular membrane.Front Cell Dev Biol. 2024 Jul 23;12:1414935. doi: 10.3389/fcell.2024.1414935. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39108834 Free PMC article. Review.
-
Conditional knockout of UBC13 produces disturbances in gait and spontaneous locomotion and exploration in mice.Sci Rep. 2019 Mar 13;9(1):4379. doi: 10.1038/s41598-019-40714-3. Sci Rep. 2019. PMID: 30867488 Free PMC article.
-
Cell-intrinsic drivers of dendrite morphogenesis.Development. 2013 Dec;140(23):4657-71. doi: 10.1242/dev.087676. Development. 2013. PMID: 24255095 Free PMC article. Review.
-
A new cellular stress response that triggers centriolar satellite reorganization and ciliogenesis.EMBO J. 2013 Nov 27;32(23):3029-40. doi: 10.1038/emboj.2013.223. Epub 2013 Oct 11. EMBO J. 2013. PMID: 24121310 Free PMC article.
-
CHARGE syndrome protein CHD7 regulates epigenomic activation of enhancers in granule cell precursors and gyrification of the cerebellum.Nat Commun. 2021 Sep 29;12(1):5702. doi: 10.1038/s41467-021-25846-3. Nat Commun. 2021. PMID: 34588434 Free PMC article.
References
-
- Altman J, Bayer S 1997. Development of the cerebellar system: In relation to its evolution, structure, and functions. CRC Press, New York
-
- Bingol B, Schuman EM 2005. Synaptic protein degradation by the ubiquitin proteasome system. Curr Opin Neurobiol 15: 536–541 - PubMed
-
- Bingol B, Schuman EM 2006. Activity-dependent dynamics and sequestration of proteasomes in dendritic spines. Nature 441: 1144–1148 - PubMed
-
- Brewer GJ 1995. Serum-free B27/neurobasal medium supports differentiated growth of neurons from the striatum, substantia nigra, septum, cerebral cortex, cerebellum, and dentate gyrus. J Neurosci Res 42: 674–683 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
