Cystic fibrosis transmembrane conductance regulator regulates epithelial cell response to Aspergillus and resultant pulmonary inflammation
- PMID: 22135344
- PMCID: PMC5448574
- DOI: 10.1164/rccm.201106-1027OC
Cystic fibrosis transmembrane conductance regulator regulates epithelial cell response to Aspergillus and resultant pulmonary inflammation
Abstract
Rationale: Mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) alter epithelial cell (EC) interactions with multiple microbes, such that dysregulated inflammation and injury occur with airway colonization in people with cystic fibrosis (CF). Aspergillus fumigatus frequently colonizes CF airways, but it has been assumed to be an innocent saprophyte; its potential role as a cause of lung disease is controversial.
Objectives: To study the interactions between Aspergillus and EC, and the role of the fungus in evoking inflammatory responses.
Methods: A. fumigatus expressing green fluorescent protein was developed for in vitro and in vivo models, which used cell lines and mouse tracheal EC.
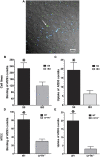
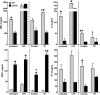
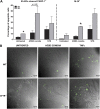
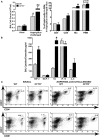
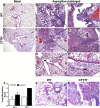
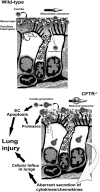
Measurements and main results: Fungal spores (conidia) are rapidly ingested by ECs derived from bronchial cell lines and murine tracheas, supporting a role for EC in early airway clearance. Bronchial ECs harboring CFTR mutations (ΔF508) or deletion demonstrate impaired uptake and killing of conidia, and ECs with CFTR mutation undergo more conidial-induced apoptosis. Germinated (hyphal) forms of the fungus evoke secretion of inflammatory mediators, with CFTR mutation resulting in increased airway levels of macrophage inflammatory protein 2 and KC, and higher lung monocyte chemotactic protein-1. After A. fumigatus inhalation, CFTR(-/-) mice develop exaggerated lymphocytic inflammation, mucin accumulation, and lung injury.
Conclusions: Data demonstrate a critical role for CFTR in mediating EC responses to A. fumigatus. Results suggest that the fungus elicits aberrant pulmonary inflammation in the setting of CFTR mutation, supporting the potential role of antifungals to halt progressive CF lung disease.
Figures


Similar articles
-
Inflammation in cystic fibrosis airways: relationship to increased bacterial adherence.Eur Respir J. 2001 Jan;17(1):27-35. doi: 10.1183/09031936.01.17100270. Eur Respir J. 2001. PMID: 11307750
-
Regulation of cystic fibrosis transmembrane conductance regulator by microRNA-145, -223, and -494 is altered in ΔF508 cystic fibrosis airway epithelium.J Immunol. 2013 Apr 1;190(7):3354-62. doi: 10.4049/jimmunol.1202960. Epub 2013 Feb 22. J Immunol. 2013. PMID: 23436935
-
Protective role of CFTR during fungal infection of cystic fibrosis bronchial epithelial cells with Aspergillus fumigatus.Front Cell Infect Microbiol. 2023 Aug 23;13:1196581. doi: 10.3389/fcimb.2023.1196581. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37680748 Free PMC article.
-
Aspergillus colonization and antifungal immunity in cystic fibrosis patients.Med Mycol. 2019 Apr 1;57(Supplement_2):S118-S126. doi: 10.1093/mmy/myy074. Med Mycol. 2019. PMID: 30816976 Review.
-
Aspergillus infections in cystic fibrosis.J Infect. 2016 Jul 5;72 Suppl:S50-5. doi: 10.1016/j.jinf.2016.04.022. Epub 2016 May 11. J Infect. 2016. PMID: 27177733 Review.
Cited by
-
The fungal airway microbiome in cystic fibrosis and non-cystic fibrosis bronchiectasis.J Cyst Fibros. 2021 Mar;20(2):295-302. doi: 10.1016/j.jcf.2020.05.013. Epub 2020 Jun 13. J Cyst Fibros. 2021. PMID: 32540174 Free PMC article.
-
Update in cystic fibrosis 2012.Am J Respir Crit Care Med. 2013 May 1;187(9):915-9. doi: 10.1164/rccm.201301-0184UP. Am J Respir Crit Care Med. 2013. PMID: 23634859 Free PMC article. Review. No abstract available.
-
Anti-Aspergillus Activities of the Respiratory Epithelium in Health and Disease.J Fungi (Basel). 2018 Jan 8;4(1):8. doi: 10.3390/jof4010008. J Fungi (Basel). 2018. PMID: 29371501 Free PMC article. Review.
-
Aspergillus fumigatus in the cystic fibrosis lung: pros and cons of azole therapy.Infect Drug Resist. 2016 Sep 20;9:229-238. doi: 10.2147/IDR.S63621. eCollection 2016. Infect Drug Resist. 2016. PMID: 27703383 Free PMC article. Review.
-
Progress in Model Systems of Cystic Fibrosis Mucosal Inflammation to Understand Aberrant Neutrophil Activity.Front Immunol. 2020 Apr 7;11:595. doi: 10.3389/fimmu.2020.00595. eCollection 2020. Front Immunol. 2020. PMID: 32318073 Free PMC article. Review.
References
-
- Teichgraber V, Ulrich M, Endlich N, Riethmuller J, Wilker B, De Oliveira-Munding CC, van Heeckeren AM, Barr ML, von Kurthy G, Schmid KW, et al. . Ceramide accumulation mediates inflammation, cell death and infection susceptibility in cystic fibrosis. Nat Med 2008;14:382–391. - PubMed
-
- Zhang Y, Li X, Grassme H, Doring G, Gulbins E. Alterations in ceramide concentration and pH determine the release of reactive oxygen species by CFTR-deficient macrophages on infection. J Immunol 2010;184:5104–5111. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

