Etsrp/Etv2 is directly regulated by Foxc1a/b in the zebrafish angioblast
- PMID: 22135404
- PMCID: PMC3457812
- DOI: 10.1161/CIRCRESAHA.111.251298
Etsrp/Etv2 is directly regulated by Foxc1a/b in the zebrafish angioblast
Abstract
Rationale: Endothelial cells are developmentally derived from angioblasts specified in the mesodermal germ cell layer. The transcription factor etsrp/etv2 is at the top of the known genetic hierarchy for angioblast development. The transcriptional events that induce etsrp expression and angioblast specification are not well understood.
Objective: We generated etsrp:gfp transgenic zebrafish and used them to identify regulatory regions and transcription factors critical for etsrp expression and angioblast specification from mesoderm.
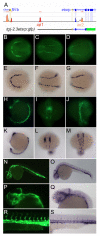
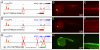
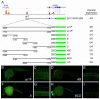
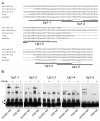
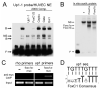
Methods and results: To investigate the mechanisms that initiate angioblast cell transcription during embryogenesis, we have performed promoter analysis of the etsrp locus in zebrafish. We describe three enhancer elements sufficient for endothelial gene expression when place in front of a heterologous promoter. The deletion of all 3 regulatory regions led to a near complete loss of endothelial expression from the etsrp promoter. One of the enhancers, located 2.3 kb upstream of etsrp contains a consensus FOX binding site that binds Foxc1a and Foxc1b in vitro by EMSA and in vivo using ChIP. Combined knockdown of foxc1a/b, using morpholinos, led to a significant decrease in etsrp expression at early developmental stages as measured by quantitative reverse transcriptase-polymerase chain reaction and in situ hybridization. Decreased expression of primitive erythrocyte genes scl and gata1 was also observed, whereas pronephric gene pax2a was relatively normal in expression level and pattern.
Conclusions: These findings identify mesodermal foxc1a/b as a direct upstream regulator of etsrp in angioblasts. This establishes a new molecular link in the process of mesoderm specification into angioblast.
Figures


Similar articles
-
Vascular endothelial and endocardial progenitors differentiate as cardiomyocytes in the absence of Etsrp/Etv2 function.Development. 2011 Nov;138(21):4721-32. doi: 10.1242/dev.064998. Development. 2011. PMID: 21989916 Free PMC article.
-
The transcription factor Foxc1a in zebrafish directly regulates expression of nkx2.5, encoding a transcriptional regulator of cardiac progenitor cells.J Biol Chem. 2018 Jan 12;293(2):638-650. doi: 10.1074/jbc.RA117.000414. Epub 2017 Nov 21. J Biol Chem. 2018. PMID: 29162723 Free PMC article.
-
Cranial vasculature in zebrafish forms by angioblast cluster-derived angiogenesis.Dev Biol. 2010 Dec 1;348(1):34-46. doi: 10.1016/j.ydbio.2010.08.036. Epub 2010 Sep 9. Dev Biol. 2010. PMID: 20832394
-
Generation and application of signaling pathway reporter lines in zebrafish.Mol Genet Genomics. 2013 Jun;288(5-6):231-42. doi: 10.1007/s00438-013-0750-z. Epub 2013 May 15. Mol Genet Genomics. 2013. PMID: 23674148 Free PMC article. Review.
-
Transcriptional control of endothelial cell development.Dev Cell. 2009 Feb;16(2):180-95. doi: 10.1016/j.devcel.2009.01.014. Dev Cell. 2009. PMID: 19217421 Free PMC article. Review.
Cited by
-
Transcriptional regulation of endothelial cell and vascular development.Circ Res. 2013 May 10;112(10):1380-400. doi: 10.1161/CIRCRESAHA.113.301078. Circ Res. 2013. PMID: 23661712 Free PMC article. Review.
-
Multiple cis-regulatory elements control prox1a expression in distinct lymphatic vascular beds.Development. 2024 May 1;151(9):dev202525. doi: 10.1242/dev.202525. Epub 2024 May 9. Development. 2024. PMID: 38722096 Free PMC article.
-
The transcription factor Mesp1 interacts with cAMP-responsive element binding protein 1 (Creb1) and coactivates Ets variant 2 (Etv2) gene expression.J Biol Chem. 2015 Apr 10;290(15):9614-25. doi: 10.1074/jbc.M114.614628. Epub 2015 Feb 18. J Biol Chem. 2015. PMID: 25694434 Free PMC article.
-
Zebrafish etv2 knock-in line labels vascular endothelial and blood progenitor cells.Dev Dyn. 2020 Feb;249(2):245-261. doi: 10.1002/dvdy.130. Epub 2019 Nov 20. Dev Dyn. 2020. PMID: 31705559 Free PMC article.
-
The regulatory role of pioneer factors during cardiovascular lineage specification - A mini review.Front Cardiovasc Med. 2022 Aug 23;9:972591. doi: 10.3389/fcvm.2022.972591. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 36082116 Free PMC article. Review.
References
-
- Stainier DY, Weinstein BM, Detrich HW, 3rd, Zon LI, Fishman MC. Cloche, an early acting zebrafish gene, is required by both the endothelial and hematopoietic lineages. Development. 1995;121:3141–3150. - PubMed
-
- Wong KS, Proulx K, Rost MS, Sumanas S. Identification of vasculature-specific genes by microarray analysis of etsrp/etv2 overexpressing zebrafish embryos. Dev Dyn. 2009;238:1836–1850. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

