Evaluating the role of connexin43 in congenital heart disease: Screening for mutations in patients with outflow tract anomalies and the analysis of knock-in mouse models
- PMID: 22135478
- PMCID: PMC3224440
- DOI: 10.4103/0975-3583.89804
Evaluating the role of connexin43 in congenital heart disease: Screening for mutations in patients with outflow tract anomalies and the analysis of knock-in mouse models
Abstract
Background: GJA1 gene encodes a gap junction protein known as connexin 43 (Cx43). Cx43 is abundantly expressed in the ventricular myocardium and in cardiac neural crest cells. Cx43 is proposed to play an important role in human congenital heart disease, as GJA1 knock-out mice die neonatally from outflow tract obstruction. In addition, patients with visceroatrial heterotaxia or hypoplastic left heart syndrome were reported to have point mutations in GJA1 at residues that affect protein kinase phosphorylation and gating of the gap junction channel. However, as these clinical findings were not replicated in subsequent studies, the question remains about the contribution of GJA1 mutations in human congenital heart disease (CHD).
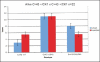
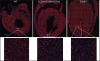
Materials and methods: We analyzed the GJA1 coding sequence in 300 patients with CHD from two clinical centers, focusing on outflow tract anomalies. This included 152 with Tetralogy of Fallot from over 200 patients exhibiting outflow tract anomalies, as well as other structural heart defects including atrioventricular septal defects and other valvar anomalies. Our sequencing analysis revealed only two silent nucleotide substitutions in 8 patients. To further assess the possible role of Cx43 in CHD, we also generated two knock-in mouse models with point mutations at serine residues subject to protein kinase C or casein kinase phosphorylation, sites that are known to regulate gating and trafficking of Cx43, respectively.
Results: Both heterozygous and homozygous knock-in mice were long term viable and did not exhibit overt CHD.
Conclusion: The combined clinical and knock-in mouse mutant studies indicate GJA1 mutation is not likely a major contributor to CHD, especially those involving outflow tract anomalies.
Keywords: Connexin43; congenital heart disease; gap junction; knock-in mouse model; outflow tract; phosphorylations.
Conflict of interest statement
Figures



Similar articles
-
On the role of the gap junction protein Cx43 (GJA1) in human cardiac malformations with Fallot-pathology. a study on paediatric cardiac specimen.PLoS One. 2014 Apr 21;9(4):e95344. doi: 10.1371/journal.pone.0095344. eCollection 2014. PLoS One. 2014. PMID: 24751918 Free PMC article.
-
Alteration in connexin 43 gap junction gene dosage impairs conotruncal heart development.Dev Biol. 1998 Jun 1;198(1):32-44. doi: 10.1006/dbio.1998.8891. Dev Biol. 1998. PMID: 9640330
-
Fate of connexin43 in cardiac tissue harbouring a disease-linked connexin43 mutant.Cardiovasc Res. 2008 Dec 1;80(3):385-95. doi: 10.1093/cvr/cvn203. Epub 2008 Aug 4. Cardiovasc Res. 2008. PMID: 18678643
-
Misregulation of connexin43 gap junction channels and congenital heart defects.Novartis Found Symp. 1999;219:212-21; discussion 221-5. doi: 10.1002/9780470515587.ch13. Novartis Found Symp. 1999. PMID: 10207906 Review.
-
Gap Junction-Dependent and -Independent Functions of Connexin43 in Biology.Biology (Basel). 2022 Feb 11;11(2):283. doi: 10.3390/biology11020283. Biology (Basel). 2022. PMID: 35205149 Free PMC article. Review.
Cited by
-
VEGFA165 gene therapy ameliorates blood-labyrinth barrier breakdown and hearing loss.JCI Insight. 2021 Apr 22;6(8):e143285. doi: 10.1172/jci.insight.143285. JCI Insight. 2021. PMID: 33690221 Free PMC article.
-
Cx43 phosphorylation-mediated effects on ERK and Akt protect against ischemia reperfusion injury and alter the stability of the stress-inducible protein NDRG1.J Biol Chem. 2019 Aug 2;294(31):11762-11771. doi: 10.1074/jbc.RA119.009162. Epub 2019 Jun 12. J Biol Chem. 2019. PMID: 31189653 Free PMC article.
-
Liganded retinoic acid X receptor α represses connexin 43 through a potential retinoic acid response element in the promoter region.Pediatr Res. 2016 Jul;80(1):159-68. doi: 10.1038/pr.2016.47. Epub 2016 Mar 18. Pediatr Res. 2016. PMID: 26991262
-
Importance of Cx43 for Right Ventricular Function.Int J Mol Sci. 2021 Jan 20;22(3):987. doi: 10.3390/ijms22030987. Int J Mol Sci. 2021. PMID: 33498172 Free PMC article. Review.
-
Spectrum of Genetic Variants in a Cohort of 37 Laterality Defect Cases.Front Genet. 2022 Apr 13;13:861236. doi: 10.3389/fgene.2022.861236. eCollection 2022. Front Genet. 2022. PMID: 35547246 Free PMC article.
References
-
- Severs N. Cardiac muscle cell interaction: From microanatomy to the molecular make-up of the gap junction. Histol Histopathol. 1995;10:481–501. - PubMed
-
- Severs N. Cardiovascular disease. Novartis Found Symp. 1999;219:188–206. discussion 206-11. - PubMed
-
- Reaume A, de Sousa P, Kulkarni S, Langille B, Zhu D, Davies T, et al. Cardiac malformation in neonatal mice lacking connexin43. Science. 1995;267:1831–4. - PubMed
-
- Li W, Waldo K, Linask K, Chen T, Wessels A, Parmacek M, et al. An essential role for connexin43 gap junctions in mouse coronary artery development. Development. 2002;129:2031–42. - PubMed
-
- Walker D, Vacha S, Kirby M, Lo C. Connexin43 deficiency causes dysregulation of coronary vasculogenesis. Dev Biol. 2005;284:479–98. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

