Analysis of the role of Ser1/Ser2/Thr9 phosphorylation on myosin II assembly and function in live cells
- PMID: 22136066
- PMCID: PMC3257205
- DOI: 10.1186/1471-2121-12-52
Analysis of the role of Ser1/Ser2/Thr9 phosphorylation on myosin II assembly and function in live cells
Erratum in
- BMC Cell Biol. 2012;13:11
Abstract
Background: Phosphorylation of non-muscle myosin II regulatory light chain (RLC) at Thr18/Ser19 is well established as a key regulatory event that controls myosin II assembly and activation, both in vitro and in living cells. RLC can also be phosphorylated at Ser1/Ser2/Thr9 by protein kinase C (PKC). Biophysical studies show that phosphorylation at these sites leads to an increase in the Km of myosin light chain kinase (MLCK) for RLC, thereby indirectly inhibiting myosin II activity. Despite unequivocal evidence that PKC phosphorylation at Ser1/Ser2/Thr9 can regulate myosin II function in vitro, there is little evidence that this mechanism regulates myosin II function in live cells.
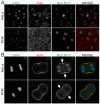
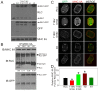
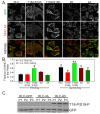
Results: The purpose of these studies was to investigate the role of Ser1/Ser2/Thr9 phosphorylation in live cells. To do this we utilized phospho-specific antibodies and created GFP-tagged RLC reporters with phosphomimetic aspartic acid substitutions or unphosphorylatable alanine substitutions at the putative inhibitory sites or the previously characterized activation sites. Cell lines stably expressing the RLC-GFP constructs were assayed for myosin recruitment during cell division, the ability to complete cell division, and myosin assembly levels under resting or spreading conditions. Our data shows that manipulation of the activation sites (Thr18/Ser19) significantly alters myosin II function in a number of these assays while manipulation of the putative inhibitory sites (Ser1/Ser2/Thr9) does not.
Conclusions: These studies suggest that inhibitory phosphorylation of RLC is not a substantial regulatory mechanism, although we cannot rule out its role in other cellular processes or perhaps other types of cells or tissues in vivo.
Figures

References
-
- Adelstein RS, Conti MA. Phosphorylation of platelet myosin increases actin-activated myosin. ATPase activity Nature. 1975;256:597–598. - PubMed
-
- Ikebe M, Hartshorne DJ, Elzinga M. Phosphorylation of the 20,000-dalton light chain of smooth muscle myosin by the calcium-activated, phospholipid-dependent protein kinase. Phosphorylation sites and effects of phosphorylation. J Biol Chem. 1987;262:9569–9573. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

