Dose effects and comparative effectiveness of extended release dexmethylphenidate and mixed amphetamine salts
- PMID: 22136094
- PMCID: PMC3243461
- DOI: 10.1089/cap.2011.0018
Dose effects and comparative effectiveness of extended release dexmethylphenidate and mixed amphetamine salts
Abstract
Objective: To compare the dose effects of long-acting extended-release dexmethylphenidate (ER d-MPH) and ER mixed amphetamine salts (ER MAS) on attention-deficit/hyperactivity disorder (ADHD) symptom dimensions, global and specific impairments, and common adverse events associated with stimulants.
Methods: Fifty-six children and adolescents with ADHD participated in an 8-week, double-blind, crossover study comparing ER d-MPH (10, 20, 25-30 mg) and ER MAS (10, 20, 25-30) with a week of randomized placebo within each drug period. Efficacy was assessed with the ADHD Rating Scale-IV (ADHD-RS-IV), whereas global and specific domains of impairment were assessed with the Clinical Global Impressions Severity and Improvement Scales and the parent-completed Weiss Functional Impairment Scale, respectively. Insomnia and decreased appetite, common stimulant-related adverse events, were measured with the parent-completed Stimulant Side Effects Rating Scale.
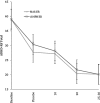
Results: Both ER d-MPH and ER MAS were associated with significant reductions in ADHD symptoms. Improvement in Total ADHD and Hyperactivity/Impulsivity symptoms were strongly associated with increasing dose, whereas improvements in Inattentive symptoms were only moderately associated with dose. About 80% demonstrated reliable change on ADHD-RS-IV at the highest dose level of ER MAS compared with 79% when receiving ER d-MPH. Decreased appetite and insomnia were more common at higher dose levels for both stimulants. Approximately 43% of the responders were preferential responders to only one of the stimulant formulations.
Conclusions: Dose level, rather than stimulant class, was strongly related to medication response.
Figures

References
-
- Achenbach TM. Manual for the Child Behavior Checklist and Revised Child Behavior Prophile. Burlington, VT: University of Vermont, Department of Psychiatry; 1991.
-
- American Academy of Child and Adolescent Psychiatry. Practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry. 2002;41:26s–48s. - PubMed
-
- American Psychiatric Association. (DSM-IV) 4th. Washington, DC: American Psychiatric Association; 1994. Diagnostic and Statistical Manual of Mental Disorders.
-
- Arnold L. Methylphenidate vs. Amphetamine: Comparative review. J Atten Disord. 2000;3:200–211.
-
- Barkley R. McMurray M. Edelbrock C. Robbins ZK. Side effects of MPH in children with attention deficit hyperactivity disorder: A systematic placebo controlled evaluation. Pediatrics. 1990;86:184–192. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

