Neuropeptide Y inhibits interleukin-1β-induced phagocytosis by microglial cells
- PMID: 22136135
- PMCID: PMC3239417
- DOI: 10.1186/1742-2094-8-169
Neuropeptide Y inhibits interleukin-1β-induced phagocytosis by microglial cells
Abstract
Background: Neuropeptide Y (NPY) is emerging as a modulator of communication between the brain and the immune system. However, in spite of increasing evidence that supports a role for NPY in the modulation of microglial cell responses to inflammatory conditions, there is no consistent information regarding the action of NPY on microglial phagocytic activity, a vital component of the inflammatory response in brain injury. Taking this into consideration, we sought to assess a potential new role for NPY as a modulator of phagocytosis by microglial cells.
Methods: The N9 murine microglial cell line was used to evaluate the role of NPY in phagocytosis. For that purpose, an IgG-opsonized latex bead assay was performed in the presence of lipopolysaccharide (LPS) and an interleukin-1β (IL-1β) challenge, and upon NPY treatment. A pharmacological approach using NPY receptor agonists and antagonists followed to uncover which NPY receptor was involved. Moreover, western blotting and immunocytochemical studies were performed to evaluate expression of p38 mitogen-activated protein kinase (MAPK) and heat shock protein 27 (HSP27), in an inflammatory context, upon NPY treatment.
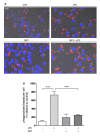
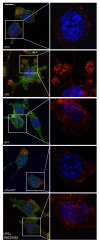
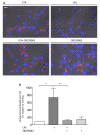
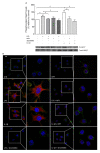
Results: Here, we show that NPY inhibits phagocytosis of opsonized latex beads and inhibits actin cytoskeleton reorganization triggered by LPS stimulation. Co-stimulation of microglia with LPS and adenosine triphosphate also resulted in increased phagocytosis, an effect inhibited by an interleukin-1 receptor antagonist, suggesting involvement of IL-1β signaling. Furthermore, direct application of LPS or IL-1β activated downstream signaling molecules, including p38 MAPK and HSP27, and these effects were inhibited by NPY. Moreover, we also observed that the inhibitory effect of NPY on phagocytosis was mediated via Y1 receptor activation.
Conclusions: Altogether, we have identified a novel role for NPY in the regulation of microglial phagocytic properties, in an inflammatory context.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

