Improved coiled-coil design enhances interaction with Bcr-Abl and induces apoptosis
- PMID: 22136227
- PMCID: PMC3313827
- DOI: 10.1021/mp200461s
Improved coiled-coil design enhances interaction with Bcr-Abl and induces apoptosis
Erratum in
-
Correction to "improved coiled-coil design enhances interaction with bcr-abl and induces apoptosis".Mol Pharm. 2012 May 7;9(5):1535. doi: 10.1021/mp300089a. Epub 2012 Mar 23. Mol Pharm. 2012. PMID: 22444272 Free PMC article. No abstract available.
Abstract
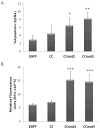
The oncoprotein Bcr-Abl drives aberrant downstream activity through trans-autophosphorylation of homo-oligomers in chronic myelogenous leukemia (CML).(1, 2) The formation of Bcr-Abl oligomers is achieved through the coiled-coil domain at the N-terminus of Bcr.(3, 4) We have previously reported a modified version of this coiled-coil domain, CCmut2, which exhibits disruption of Bcr-Abl oligomeric complexes and results in decreased proliferation of CML cells and induction of apoptosis.(5) A major contributing factor to these enhanced capabilities is the destabilization of the CCmut2 homodimers, increasing the availability to interact with and inhibit Bcr-Abl. Here, we included an additional mutation (K39E) that could in turn further destabilize the mutant homodimer. Incorporation of this modification into CCmut2 (C38A, S41R, L45D, E48R, Q60E) generated what we termed CCmut3, and resulted in further improvements in the binding properties with the wild-type coiled-coil domain representative of Bcr-Abl [corrected]. A separate construct containing one revert mutation, CCmut4, did not demonstrate improved oligomeric properties and indicated the importance of the L45D mutation. CCmut3 demonstrated improved oligomerization via a two-hybrid assay as well as through colocalization studies, in addition to showing similar biologic activity as CCmut2. The improved binding between CCmut3 and the Bcr-Abl coiled-coil may be used to redirect Bcr-Abl to alternative subcellular locations with interesting therapeutic implications.
Figures

References
-
- Guo XY, Cuillerot JM, Wang T, Wu Y, Arlinghaus R, Claxton D, Bachier C, Greenberger J, Colombowala I, Deisseroth AB. Peptide containing the BCR oligomerization domain (AA 1-160) reverses the transformed phenotype of p210bcr-abl positive 32D myeloid leukemia cells. Oncogene. 1998;17(7):825–33. - PubMed
-
- Zhao X, Ghaffari S, Lodish H, Malashkevich VN, Kim PS. Structure of the Bcr-Abl oncoprotein oligomerization domain. Nat Struct Biol. 2002;9(2):117–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

