Influence of the Chungkookjang on histamine-induced wheal and flare skin response: a randomized, double-blind, placebo controlled trial
- PMID: 22136279
- PMCID: PMC3295693
- DOI: 10.1186/1472-6882-11-125
Influence of the Chungkookjang on histamine-induced wheal and flare skin response: a randomized, double-blind, placebo controlled trial
Abstract
Background: Allergic disease is a consequence of exposure to normally innocuous substances that elicit the activation of mast cells. Mast-cell-mediated allergic response is involved in many diseases such as anaphylaxis, urticaria, allergic rhinitis, asthma and allergic dermatitis. The development of food products for the prevention of allergic disease is an important subject in human health. The chungkookjang (CKJ) has been reported to exhibit antiallergic inflammatory activity. Therefore, the aim of the study is to examine the effects of the CKJ to reduce histamine-induced wheal and flare skin responses.
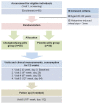
Methods/design: A randomized, double-blind, placebo-controlled study in 60 healthy subjects will be carried out. Sixty volunteers (aged 20-80) who gave a written consent before entering the study will be randomized in two groups of thirty subjects each. The skin prick test with histamine solution of 10 mg/ml will be performed on the ventral forearm, 10 cm from the elbow. The subjects will be instructed to take 35 g per day of either the CKJ pills or a placebo pills for a period of 3 months. Diameters of wheal and flare will be assessing 15 minutes after performing the above-mentioned skin prick test. The primary outcome is change in wheal and flare responses. Secondary outcomes will be include change in serum histamine, immunoglobulin E, cytokines (interferon-gamma, interleukin-4, -10, and tumor necrosis factor-alpha), and eosinophil cationic protein.
Discussion: This study will show the potential anti-inflammatory properties of the CKJ in their skin activity when histamine is the challenging agent as occurs in the clinical situation. And the present protocol will confirm the efficacy and safety of the CKJ for allergy symptoms, suggesting more basic knowledge to conduct further randomized controlled trials (RCT). If this study will be successfully performed, the CKJ will be an alternative dietary supplemental remedy for allergy patients.
Trial registration: NCT01402141.
Figures
Similar articles
-
[Comparative activity of antihistamines on area under dose-response curve from histamine-induced wheal and flare responses in human skin].Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2004 Dec;26(6):634-8. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2004. PMID: 15663222 Clinical Trial. Chinese.
-
Comparative activity of cetirizine and mizolastine on histamine-induced skin wheal and flare responses at 24 h.Br J Clin Pharmacol. 2002 Mar;53(3):250-4. doi: 10.1046/j.0306-5251.2001.01551.x. Br J Clin Pharmacol. 2002. PMID: 11874388 Free PMC article. Clinical Trial.
-
Cetirizine inhibits bradykinin-induced cutaneous wheal and flare in atopic and healthy subjects.Allergy. 2000 Sep;55(9):888-91. doi: 10.1034/j.1398-9995.2000.00322.x. Allergy. 2000. PMID: 11003455 Clinical Trial.
-
Appraisal of the validity of histamine-induced wheal and flare to predict the clinical efficacy of antihistamines.J Allergy Clin Immunol. 1997 Feb;99(2):S798-806. doi: 10.1016/s0091-6749(97)70128-3. J Allergy Clin Immunol. 1997. PMID: 9042073 Review.
-
Antiallergic effects of H1-receptor antagonists.Allergy. 2000;55 Suppl 64:17-27. doi: 10.1034/j.1398-9995.2000.00803.x. Allergy. 2000. PMID: 11291777 Review.
Cited by
-
Specific oligopeptides in fermented soybean extract inhibit NF-κB-dependent iNOS and cytokine induction by toll-like receptor ligands.J Med Food. 2014 Nov;17(11):1239-46. doi: 10.1089/jmf.2013.3070. Epub 2014 Sep 3. J Med Food. 2014. PMID: 25184943 Free PMC article.
-
Cheonggukjang ethanol extracts inhibit a murine allergic asthma via suppression of mast cell-dependent anaphylactic reactions.J Med Food. 2014 Jan;17(1):142-9. doi: 10.1089/jmf.2013.2997. J Med Food. 2014. PMID: 24456365 Free PMC article.
References
-
- Marshall JS. Mast-cell responses to pathogens. Nat Rev Immunol. 2004;4(10):787–799. - PubMed
-
- Rivera J, Gilfillan AM. Molecular regulation of mast cell activation. J Alergy Clin Immunol. 2006;117(6):1214–1225. quiz 1226. - PubMed
-
- Kawata R, Reddy ST, Wolner B, Herschman HR. Prostaglandin synthase 1 and prostaglandin synthase 2 both participate in activation-induced prostaglandin D2 production in mast cells. J Immunol. 1995;155(2):818–825. - PubMed
-
- Moon TC, Murakami M, Ashraf MD, Kudo I, Chang HW. Regulation of cyclooxygenase-2 and endogenous cytokine expression by bacterial lipopolysaccharide that acts in synergy with c-kit ligand and Fc epsilon receptor I crosslinking in cultured mast cells. Cell Immunol. 1998;185(2):146–152. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical