Broad substrate specificity of the amide synthase in S. hygroscopicus--new 20-membered macrolactones derived from geldanamycin
- PMID: 22136518
- PMCID: PMC3292439
- DOI: 10.1021/ja2087147
Broad substrate specificity of the amide synthase in S. hygroscopicus--new 20-membered macrolactones derived from geldanamycin
Abstract
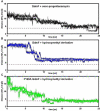
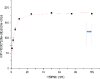
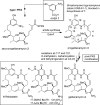
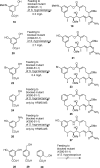
The amide synthase of the geldanamycin producer, Streptomyces hygroscopicus, shows a broader chemoselectivity than the corresponding amide synthase present in Actinosynnema pretiosum, the producer of the highly cytotoxic ansamycin antibiotics, the ansamitocins. This was demonstrated when blocked mutants of both strains incapable of biosynthesizing 3-amino-5-hydroxybenzoic acid (AHBA), the polyketide synthase starter unit of both natural products, were supplemented with 3-amino-5-hydroxymethylbenzoic acid instead. Unlike the ansamitocin producer A. pretiosum, S. hygroscopicus processed this modified starter unit not only to the expected 19-membered macrolactams but also to ring enlarged 20-membered macrolactones. The former mutaproducts revealed the sequence of transformations catalyzed by the post-PKS tailoring enzymes in geldanamycin biosynthesis. The unprecedented formation of the macrolactones together with molecular modeling studies shed light on the mode of action of the amide synthase responsible for macrocyclization. Obviously, the 3-hydroxymethyl substituent shows similar reactivity and accessibility toward C-1 of the seco-acid as the arylamino group, while phenolic hydroxyl groups lack this propensity to act as nucleophiles in the macrocyclization. The promiscuity of the amide synthase of S. hygroscopicus was further demonstrated by successful feeding of four other m-hydroxymethylbenzoic acids, leading to formation of the expected 20-membered macrocycles. Good to moderate antiproliferative activities were encountered for three of the five new geldanamycin derivatives, which matched well with a competition assay for Hsp90α.
© 2011 American Chemical Society
Figures




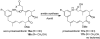
References
-
- Workman P. Curr. Cancer Drug Targets. 2003;3:297–300. - PubMed
- Neckers L, Neckers K. Expert Opin. Emerg. Drugs. 2005;10:137–149. - PubMed
- Whitesell L, Lindquist SL. Nat. Rev. Cancer. 2005;5:761–772. - PubMed
- Prodromou C, Roe SM, O'Brien R, Ladbury JE, Piper PW, Pearl LH. Cell. 1997;90:65–75. - PubMed
-
-
Review: Biamonte MA, van de Water R, Arndt JW, Scannevin RH, Perret D, Lee W-C. J. Med. Chem. 2010;53:3–17..
-
-
- Blagosklonny MV, Toretsky J, Neckers L. Oncogene. 1995;11:933–939. - PubMed
-
- Janin L. J. Med. Chem. 2005;48:7503–7512. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

