Bacterial isolation by lectin-modified microengines
- PMID: 22136558
- PMCID: PMC3256279
- DOI: 10.1021/nl203717q
Bacterial isolation by lectin-modified microengines
Abstract
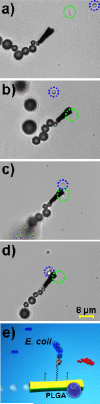
New template-based self-propelled gold/nickel/polyaniline/platinum (Au/Ni/PANI/Pt) microtubular engines, functionalized with the Concanavalin A (ConA) lectin bioreceptor, are shown to be extremely useful for the rapid, real-time isolation of Escherichia coli (E. coli) bacteria from fuel-enhanced environmental, food, and clinical samples. These multifunctional microtube engines combine the selective capture of E. coli with the uptake of polymeric drug-carrier particles to provide an attractive motion-based theranostics strategy. Triggered release of the captured bacteria is demonstrated by movement through a low-pH glycine-based dissociation solution. The smaller size of the new polymer-metal microengines offers convenient, direct, and label-free optical visualization of the captured bacteria and discrimination against nontarget cells.
© 2011 American Chemical Society
Figures



Similar articles
-
Identification of Critical Surface Parameters Driving Lectin-Mediated Capture of Bacteria from Solution.Biomacromolecules. 2019 Jul 8;20(7):2852-2863. doi: 10.1021/acs.biomac.9b00609. Epub 2019 Jun 17. Biomacromolecules. 2019. PMID: 31150217
-
A lectin-coupled porous silicon-based biosensor: label-free optical detection of bacteria in a real-time mode.Sci Rep. 2020 Sep 29;10(1):16017. doi: 10.1038/s41598-020-72457-x. Sci Rep. 2020. PMID: 32994483 Free PMC article.
-
Functionalized ultrasound-propelled magnetically guided nanomotors: toward practical biomedical applications.ACS Nano. 2013 Oct 22;7(10):9232-40. doi: 10.1021/nn403851v. Epub 2013 Aug 28. ACS Nano. 2013. PMID: 23971861
-
Supramolecular assembly/reassembly processes: molecular motors and dynamers operating at surfaces.Nanoscale. 2011 Apr;3(4):1397-410. doi: 10.1039/c0nr00914h. Epub 2011 Feb 24. Nanoscale. 2011. PMID: 21350766 Review.
-
Molecular rotors and motors: recent advances and future challenges.ACS Nano. 2009 May 26;3(5):1042-8. doi: 10.1021/nn900411n. ACS Nano. 2009. PMID: 19845364 Review.
Cited by
-
Light-Powered Micro/Nanomotors.Micromachines (Basel). 2018 Jan 23;9(2):41. doi: 10.3390/mi9020041. Micromachines (Basel). 2018. PMID: 30393317 Free PMC article. Review.
-
Nanoscale Biosensors Based on Self-Propelled Objects.Biosensors (Basel). 2018 Jun 25;8(3):59. doi: 10.3390/bios8030059. Biosensors (Basel). 2018. PMID: 29941799 Free PMC article. Review.
-
Catalytically powered dynamic assembly of rod-shaped nanomotors and passive tracer particles.Proc Natl Acad Sci U S A. 2013 Oct 29;110(44):17744-9. doi: 10.1073/pnas.1311543110. Epub 2013 Oct 14. Proc Natl Acad Sci U S A. 2013. PMID: 24127603 Free PMC article.
-
Nanomaterials for Biosensing Lipopolysaccharide.Biosensors (Basel). 2019 Dec 21;10(1):2. doi: 10.3390/bios10010002. Biosensors (Basel). 2019. PMID: 31877825 Free PMC article. Review.
-
Dual-Fuel-Driven Bactericidal Micromotor.Nanomicro Lett. 2016;8(2):157-164. doi: 10.1007/s40820-015-0071-3. Epub 2015 Nov 13. Nanomicro Lett. 2016. PMID: 30460276 Free PMC article.
References
-
- Laurino P, Kikkeri R. Nano Lett. 2011;11:73–78. - PubMed
-
- Guven B, Basaran-Akgul N, Temur E, Tamer U, Boyacı IH. Analyst. 2011;136:740–748. - PubMed
-
- Wang R, Ruan C, Kanayeva D, Lassiter K, Li Y. Nano Lett. 2008;8:2625–2635. - PubMed
-
- Arya SK, Singh A, Naidoo R, Wu P, McDermott MT, Evoy S. Analyst. 2011;136:486–492. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

