Rationale for the diabetic retinopathy clinical research network treatment protocol for center-involved diabetic macular edema
- PMID: 22136692
- PMCID: PMC3253487
- DOI: 10.1016/j.ophtha.2011.09.058
Rationale for the diabetic retinopathy clinical research network treatment protocol for center-involved diabetic macular edema
Abstract
Objective: To describe the underlying principles used to develop a web-based algorithm that incorporated intravitreal anti-vascular endothelial growth factor (anti-VEGF) treatment for diabetic macular edema (DME) in a Diabetic Retinopathy Clinical Research Network (DRCR.net) randomized clinical trial.
Design: Discussion of treatment protocol for DME.
Participants: Subjects with vision loss resulting from DME involving the center of the macula.
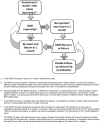
Methods: The DRCR.net created an algorithm incorporating anti-VEGF injections in a comparative effectiveness randomized clinical trial evaluating intravitreal ranibizumab with prompt or deferred (≥24 weeks) focal/grid laser treatment in eyes with vision loss resulting from center-involved DME. Results confirmed that intravitreal ranibizumab with prompt or deferred laser provides superior visual acuity outcomes compared with prompt laser alone through at least 2 years. Duplication of this algorithm may not be practical for clinical practice. To share their opinion on how ophthalmologists might emulate the study protocol, participating DRCR.net investigators developed guidelines based on the algorithm's underlying rationale.
Main outcome measures: Clinical guidelines based on a DRCR.net protocol.
Results: The treatment protocol required real-time feedback from a web-based data entry system for intravitreal injections, focal/grid laser treatment, and follow-up intervals. Guidance from this system indicated whether treatment was required or given at investigator discretion and when follow-up should be scheduled. Clinical treatment guidelines, based on the underlying clinical rationale of the DRCR.net protocol, include repeating treatment monthly as long as there is improvement in edema compared with the previous month or until the retina is no longer thickened. If thickening recurs or worsens after discontinuing treatment, treatment is resumed.
Conclusions: Duplication of the approach used in the DRCR.net randomized clinical trial to treat DME involving the center of the macula with intravitreal ranibizumab may not be practical in clinical practice, but likely can be emulated based on an understanding of the underlying rationale for the study protocol. Inherent differences between a web-based treatment algorithm and a clinical approach may lead to differences in outcomes that are impossible to predict. The closer the clinical approach is to the algorithm used in the study, the more likely the outcomes will be similar to those published.
Financial disclosure(s): Proprietary or commercial disclosure may be found after the references.
Copyright © 2011 American Academy of Ophthalmology. Published by Elsevier Inc. All rights reserved.
Figures





Similar articles
-
Intravitreal Ranibizumab for diabetic macular edema with prompt versus deferred laser treatment: 5-year randomized trial results.Ophthalmology. 2015 Feb;122(2):375-81. doi: 10.1016/j.ophtha.2014.08.047. Epub 2014 Oct 28. Ophthalmology. 2015. PMID: 25439614 Free PMC article. Clinical Trial.
-
Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema.Ophthalmology. 2010 Jun;117(6):1064-1077.e35. doi: 10.1016/j.ophtha.2010.02.031. Epub 2010 Apr 28. Ophthalmology. 2010. PMID: 20427088 Free PMC article. Clinical Trial.
-
Intravitreal ranibizumab for diabetic macular edema with prompt versus deferred laser treatment: three-year randomized trial results.Ophthalmology. 2012 Nov;119(11):2312-8. doi: 10.1016/j.ophtha.2012.08.022. Epub 2012 Sep 19. Ophthalmology. 2012. PMID: 22999634 Free PMC article. Clinical Trial.
-
Practical Lessons from Protocol I for the Management of Diabetic Macular Edema.Dev Ophthalmol. 2017;60:91-108. doi: 10.1159/000459692. Epub 2017 Apr 20. Dev Ophthalmol. 2017. PMID: 28427069 Review.
-
Vascular endothelial growth factor and diabetic macular edema.Surv Ophthalmol. 2016 Nov-Dec;61(6):759-768. doi: 10.1016/j.survophthal.2016.03.010. Epub 2016 Apr 1. Surv Ophthalmol. 2016. PMID: 27045225 Review.
Cited by
-
Association between cystoid spaces on indocyanine green hyperfluorescence and optical coherence tomography after vitrectomy for diabetic macular oedema.Eye (Lond). 2014 Apr;28(4):439-48. doi: 10.1038/eye.2013.290. Epub 2014 Jan 24. Eye (Lond). 2014. PMID: 24458201 Free PMC article.
-
Intravitreal injection of methotrexate in persistent diabetic macular edema: a 6-month follow-up study.Graefes Arch Clin Exp Ophthalmol. 2016 Nov;254(11):2159-2164. doi: 10.1007/s00417-016-3374-2. Epub 2016 May 5. Graefes Arch Clin Exp Ophthalmol. 2016. PMID: 27145784
-
Identification of time point to best define 'sub-optimal response' following intravitreal ranibizumab therapy for diabetic macular edema based on real-life data.Eye (Lond). 2017 Nov;31(11):1594-1599. doi: 10.1038/eye.2017.111. Epub 2017 Jun 16. Eye (Lond). 2017. PMID: 28622321 Free PMC article.
-
Efficacy and Safety of a Dexamethasone Implant in Patients with Diabetic Macular Edema at Tertiary Centers in Korea.J Ophthalmol. 2016;2016:9810270. doi: 10.1155/2016/9810270. Epub 2016 May 17. J Ophthalmol. 2016. PMID: 27293879 Free PMC article.
-
Revolution to a new standard treatment of diabetic macular edema.JAMA. 2014 Jun 11;311(22):2269-70. doi: 10.1001/jama.2014.2536. JAMA. 2014. PMID: 24915254 Free PMC article. No abstract available.
References
-
- Brown JC, Solomon SD, Bressler SB, et al. Detection of diabetic foveal edema: contact lens biomicroscopy compared with optical coherence tomography. Arch Ophthalmol. 2004;122:330–5. - PubMed
-
- Browning DJ, McOwen MD, Bowen RM, Jr, O'Marah TL. Comparison of the clinical diagnosis of diabetic macular edema with diagnosis by optical coherence tomography. Ophthalmology. 2004;111:712–5. - PubMed
-
- Aiello LP, Brucker AJ, Chang S, et al. Evolving guidelines for intravitreous injections. Retina. 2004;24(suppl):S3–19. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

