Developing mechanistic insights into cardiovascular cell therapy: Cardiovascular Cell Therapy Research Network Biorepository Core Laboratory rationale
- PMID: 22137069
- PMCID: PMC3236502
- DOI: 10.1016/j.ahj.2011.05.024
Developing mechanistic insights into cardiovascular cell therapy: Cardiovascular Cell Therapy Research Network Biorepository Core Laboratory rationale
Abstract
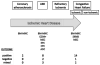
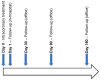
Moderate improvements in cardiac performance have been reported in some clinical settings after delivery of bone marrow mononuclear cells to patients with cardiovascular disease. However, mechanistic insights into how these cells impact outcomes are lacking. To address this, the National Heart, Lung and Blood Institute (NHLBI) Cardiovascular Cell Therapy Research Network (CCTRN) established a Biorepository Core for extensive phenotyping and cell function studies and storing bone marrow and peripheral blood for 10 years. Analyzing cell populations and cell function in the context of clinical parameters and clinical outcomes after cell or placebo treatment empower the development of novel diagnostic and prognostics. Developing such biomarkers that define the safety and efficacy of cell therapy is a major Biorepository aim.
Copyright © 2011 Mosby, Inc. All rights reserved.
Conflict of interest statement
Figures




References
-
- Abdel-Latif A, Bolli R, Tleyjeh IM, Montori VM, Perin EC, Hornung CA, Zuba-Surma EK, Al-Mallah M, Dawn B. Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Arch Intern Med. 2007;167(10):989–997. - PubMed
-
- Lipinski MJ, Biondi-Zoccai GG, Abbate A, Khianey R, Sheiban I, Bartunek J, Vanderheyden M, Kim HS, Kang HJ, Strauer BE, Vetrovec GW. Impact of intracoronary cell therapy on left ventricular function in the setting of acute myocardial infarction: a collaborative systematic review and meta-analysis of controlled clinical trials. J Am Coll Cardiol. 2007;50(18):1761–1767. - PubMed
-
- Losordo DW, Schatz RA, White CJ, Udelson JE, Veereshwarayya V, Durgin M, Poh KK, Weinstein R, Kearney M, Chaudhry M, Burg A, Eaton L, Heyd L, Thorne T, Shturman L, Hoffmeister P, Story K, Zak V, Dowling D, Traverse JH, Olson RE, Flanagan J, Sodano D, Murayama T, Kawamoto A, Kusano KF, Wollins J, Welt F, Shah P, Soukas P, Asahara T, Henry TD. Intramyocardial transplantation of autologous CD34+ stem cells for intractable angina: a phase I/IIa double-blind, randomized controlled trial. Circulation. 2007;115(25):3165–3172. - PubMed
-
- Schächinger V, Erbs S, Elsässer A, Haberbosch W, Hambrecht R, Hölschermann H, Yu J, Corti R, Mathey DG, Hamm CW, Süselbeck T, Werner N, Haase J, Neuzner J, Germing A, Mark B, Assmus B, Tonn T, Dimmeler S, Zeiher AM REPAIR-AMI Investigators. Improved clinical outcome after intracoronary administration of bone-marrow-derived progenitor cells in acute myocardial infarction: final 1-year results of the REPAIR-AMI trial. Eur Heart J. 2006;27(23):2775–2783. - PubMed
-
- van Ramshorst J, Bax JJ, Beeres SL, Dibbets-Schneider P, Roes SD, Stokkel MP, de Roos A, Fibbe WE, Zwaginga JJ, Boersma E, Schalij MJ, Atsma DE. Intramyocardial bone marrow cell injection for chronic myocardial ischemia: a randomized controlled trial. JAMA. 2009;301(19):1997–2004. - PubMed

