Phase 2 study of neoadjuvant treatment with NOV-002 in combination with doxorubicin and cyclophosphamide followed by docetaxel in patients with HER-2 negative clinical stage II-IIIc breast cancer
- PMID: 22138748
- PMCID: PMC3697917
- DOI: 10.1007/s10549-011-1889-0
Phase 2 study of neoadjuvant treatment with NOV-002 in combination with doxorubicin and cyclophosphamide followed by docetaxel in patients with HER-2 negative clinical stage II-IIIc breast cancer
Abstract
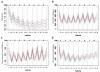
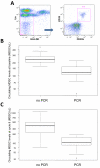
NOV-002 (a formulation of disodium glutathione disulfide) modulates signaling pathways involved in tumor cell proliferation and metastasis and enhances anti-tumor immune responsiveness in tumor models. The addition of NOV-002 to chemotherapy has been shown to increase anti-tumor efficacy in animal models and some early phase oncology trials. We evaluated the clinical effects of NOV-002 in primary breast cancer, whether adding NOV-002 to standard preoperative chemotherapy increased pathologic complete response rates (pCR) at surgery, and determined whether NOV-002 mitigated hematologic toxicities of chemotherapy and whether levels of myeloid derived suppressor cells (MDSC) were predictive of response. Forty-one women with newly diagnosed stages II-IIIc HER-2 negative breast cancer received doxorubicin-cyclophosphamide followed by docetaxel (AC → T) every 3 weeks and concurrent daily NOV-002 injections. The trial was powered to detect a doubling of pCR rate from 16 to 32% with NOV-002 plus AC → T (α = 0.05, β = 80%). Weekly complete blood counts were obtained as well as circulating MDSC levels on day 1 of each cycle were quantified. Of 39 patients with 40 evaluable tumors, 15 achieved a pCR (38%), meeting the primary endpoint of the trial. Concurrent NOV-002 resulted in pCR rates for AC → T chemotherapy higher than previously reported. Patients with lower levels of circulating MDSCs at baseline and on the last cycle of chemotherapy had significantly higher probability of a pCR (P = 0.02). Further evaluation of NOV-002 in a randomized study is warranted.
Figures
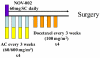

Similar articles
-
A randomized phase II trial of doxorubicin plus pemetrexed followed by docetaxel versus doxorubicin plus cyclophosphamide followed by docetaxel as neoadjuvant treatment of early breast cancer.Ann Oncol. 2011 Mar;22(3):609-617. doi: 10.1093/annonc/mdq400. Epub 2010 Aug 23. Ann Oncol. 2011. PMID: 20732932 Clinical Trial.
-
HER-2/neu expression as a predictor of response to neoadjuvant docetaxel in patients with operable breast carcinoma.Cancer. 2005 Jun 1;103(11):2252-60. doi: 10.1002/cncr.21037. Cancer. 2005. PMID: 15834928 Clinical Trial.
-
Prospective phase II study of neoadjuvant doxorubicin followed by cisplatin/docetaxel in locally advanced breast cancer.Med Oncol. 2010 Sep;27(3):571-7. doi: 10.1007/s12032-009-9251-7. Epub 2009 Jun 13. Med Oncol. 2010. PMID: 19526202 Clinical Trial.
-
Phase II study of neoadjuvant docetaxel/ vinorelbine followed by surgery and adjuvant doxorubicin/cyclophosphamide in women with stage II/III breast cancer.Clin Breast Cancer. 2006 Feb;6(6):511-7. doi: 10.3816/CBC.2006.n.004. Clin Breast Cancer. 2006. PMID: 16595034 Clinical Trial.
-
Concurrent bevacizumab with a sequential regimen of doxorubicin and cyclophosphamide followed by docetaxel and capecitabine as neoadjuvant therapy for HER2- locally advanced breast cancer: a phase II trial of the NSABP Foundation Research Group.Clin Breast Cancer. 2011 Aug;11(4):228-34. doi: 10.1016/j.clbc.2011.04.001. Epub 2011 May 4. Clin Breast Cancer. 2011. PMID: 21684812 Clinical Trial.
Cited by
-
ROS and the DNA damage response in cancer.Redox Biol. 2019 Jul;25:101084. doi: 10.1016/j.redox.2018.101084. Epub 2018 Dec 21. Redox Biol. 2019. PMID: 30612957 Free PMC article. Review.
-
Redox Paradox: A Novel Approach to Therapeutics-Resistant Cancer.Antioxid Redox Signal. 2018 Nov 1;29(13):1237-1272. doi: 10.1089/ars.2017.7485. Epub 2018 Feb 21. Antioxid Redox Signal. 2018. PMID: 29325444 Free PMC article. Review.
-
Clinical evaluation of systemic and local immune responses in cancer: time for integration.Cancer Immunol Immunother. 2014 Jan;63(1):45-57. doi: 10.1007/s00262-013-1480-0. Epub 2013 Oct 8. Cancer Immunol Immunother. 2014. PMID: 24100804 Free PMC article.
-
Oxidative stress, redox regulation and diseases of cellular differentiation.Biochim Biophys Acta. 2015 Aug;1850(8):1607-21. doi: 10.1016/j.bbagen.2014.11.010. Epub 2014 Nov 15. Biochim Biophys Acta. 2015. PMID: 25445706 Free PMC article. Review.
-
Novel Pathways of Oxidative and Nitrosative Inactivation of the Human MGMT Protein in Colon Cancer and Glioblastoma Cells: Increased Efficacy of Alkylating Agents In Vitro and In Vivo.Diseases. 2025 Jan 25;13(2):32. doi: 10.3390/diseases13020032. Diseases. 2025. PMID: 39997039 Free PMC article.
References
-
- Montero AJ, Jassem J. Cellular redox pathways as a therapeutic target in the treatment of cancer. Drugs. 2011;71(11):1385–1396. - PubMed
-
- Irani K, Xia Y, Zweier JL, Sollott SJ, Der CJ, Fearon ER, Sundaresan M, Finkel T, Goldschmidt-Clermont PJ. Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Science (New York, NY) 1997;275(5306):1649–1652. - PubMed
-
- Laurent A, Nicco C, Chereau C, Goulvestre C, Alexandre J, Alves A, Levy E, Goldwasser F, Panis Y, Soubrane O, et al. Controlling tumor growth by modulating endogenous production of reactive oxygen species. Cancer research. 2005;65(3):948–956. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

