Inhibitors of testosterone biosynthetic and metabolic activation enzymes
- PMID: 22138857
- PMCID: PMC6264586
- DOI: 10.3390/molecules16129983
Inhibitors of testosterone biosynthetic and metabolic activation enzymes
Abstract
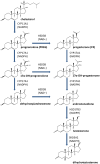
The Leydig cells of the testis have the capacity to biosynthesize testosterone from cholesterol. Testosterone and its metabolically activated product dihydrotestosterone are critical for the development of male reproductive system and spermatogenesis. At least four steroidogenic enzymes are involved in testosterone biosynthesis: Cholesterol side chain cleavage enzyme (CYP11A1) for the conversion of cholesterol into pregnenolone within the mitochondria, 3β-hydroxysteroid dehydrogenase (HSD3B), for the conversion of pregnenolone into progesterone, 17α-hydroxylase/17,20-lyase (CYP17A1) for the conversion of progesterone into androstenedione and 17β-hydroxysteroid dehydrogenase (HSD17B3) for the formation of testosterone from androstenedione. Testosterone is also metabolically activated into more potent androgen dihydrotestosterone by two isoforms 5α-reductase 1 (SRD5A1) and 2 (SRD5A2) in Leydig cells and peripheral tissues. Many endocrine disruptors act as antiandrogens via directly inhibiting one or more enzymes for testosterone biosynthesis and metabolic activation. These chemicals include industrial materials (perfluoroalkyl compounds, phthalates, bisphenol A and benzophenone) and pesticides/biocides (methoxychlor, organotins, 1,2-dibromo-3-chloropropane and prochloraz) and plant constituents (genistein and gossypol). This paper reviews these endocrine disruptors targeting steroidogenic enzymes.
Figures
Similar articles
-
Environmental pollutants and hydroxysteroid dehydrogenases.Vitam Horm. 2014;94:349-90. doi: 10.1016/B978-0-12-800095-3.00013-4. Vitam Horm. 2014. PMID: 24388197 Review.
-
Differential effects of octylphenol, 17beta-estradiol, endosulfan, or bisphenol A on the steroidogenic competence of cultured adult rat Leydig cells.Reprod Toxicol. 2001 Sep-Oct;15(5):551-60. doi: 10.1016/s0890-6238(01)00158-7. Reprod Toxicol. 2001. PMID: 11780963
-
Effects of dexmedetomidine on the steroidogenesis of rat immature Leydig cells.Steroids. 2019 Sep;149:108423. doi: 10.1016/j.steroids.2019.05.015. Epub 2019 Jun 6. Steroids. 2019. PMID: 31175921
-
Endocrine disruptors of inhibiting testicular 3β-hydroxysteroid dehydrogenase.Chem Biol Interact. 2019 Apr 25;303:90-97. doi: 10.1016/j.cbi.2019.02.027. Epub 2019 Feb 28. Chem Biol Interact. 2019. PMID: 30826252 Review.
-
Assessment of steroidogenesis and steroidogenic enzyme functions.J Steroid Biochem Mol Biol. 2013 Sep;137:176-82. doi: 10.1016/j.jsbmb.2013.05.017. Epub 2013 Jun 13. J Steroid Biochem Mol Biol. 2013. PMID: 23770321 Review.
Cited by
-
The Effects of Chronic Lead Exposure on Testicular Development of Japanese Quail (Coturnix japonica): Histopathological Damages, Oxidative Stress, Steroidogenesis Disturbance, and Hypothalamus-Pituitary-Testis Axis Disruption.Biol Trace Elem Res. 2023 Jul;201(7):3446-3460. doi: 10.1007/s12011-022-03436-8. Epub 2022 Oct 10. Biol Trace Elem Res. 2023. PMID: 36210404
-
Perfluoroalkyl Chemicals and Male Reproductive Health: Do PFOA and PFOS Increase Risk for Male Infertility?Int J Environ Res Public Health. 2021 Apr 5;18(7):3794. doi: 10.3390/ijerph18073794. Int J Environ Res Public Health. 2021. PMID: 33916482 Free PMC article. Review.
-
Testicular fat deposition attenuates reproductive performance via decreased follicle-stimulating hormone level and sperm meiosis and testosterone synthesis in mouse.Anim Biosci. 2024 Jan;37(1):50-60. doi: 10.5713/ab.23.0175. Epub 2023 Aug 28. Anim Biosci. 2024. PMID: 37641828 Free PMC article.
-
Assessment of the Emerging Threat Posed by Perfluoroalkyl and Polyfluoroalkyl Substances to Male Reproduction in Humans.Front Endocrinol (Lausanne). 2022 Mar 9;12:799043. doi: 10.3389/fendo.2021.799043. eCollection 2021. Front Endocrinol (Lausanne). 2022. PMID: 35356147 Free PMC article. Review.
-
Dietary quercetin and vitamin E supplementation modulates the reproductive performance and antioxidant capacity of aged male breeder chickens.Poult Sci. 2022 Jun;101(6):101851. doi: 10.1016/j.psj.2022.101851. Epub 2022 Mar 11. Poult Sci. 2022. PMID: 35472738 Free PMC article.
References
-
- Huhtaniemi I., Pelliniemi L.J. Fetal Leydig cells: Cellular origin, morphology, life span, and special functional features. Proc. Soc. Exp. Biol. Med. 1992;201:125–140. - PubMed
-
- Awoniyi C.A., Santulli R., Sprando R.L., Ewing L.L., Zirkin B.R. Restoration of advanced spermatogenic cells in the experimentally regressed rat testis: Quantitative relationship to testosterone concentration within the testis. Endocrinology. 1989;124:1217–1223. doi: 10.1210/endo-124-3-1217. - DOI - PubMed
-
- Fujii T. Roles of age and androgen in the regulation of sex accessory organs. Adv. Sex Horm. Res. 1977;3:103–137. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources