Sperm preparation: state-of-the-art--physiological aspects and application of advanced sperm preparation methods
- PMID: 22138904
- PMCID: PMC3735088
- DOI: 10.1038/aja.2011.133
Sperm preparation: state-of-the-art--physiological aspects and application of advanced sperm preparation methods
Abstract
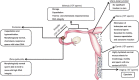
For assisted reproduction technologies (ART), numerous techniques were developed to isolate spermatozoa capable of fertilizing oocytes. While early methodologies only focused on isolating viable, motile spermatozoa, with progress of ART, particularly intracytoplasmic sperm injection (ICSI), it became clear that these parameters are insufficient for the identification of the most suitable spermatozoon for fertilization. Conventional sperm preparation techniques, namely, swim-up, density gradient centrifugation and glass wool filtration, are not efficient enough to produce sperm populations free of DNA damage, because these techniques are not physiological and not modeled on the stringent sperm selection processes taking place in the female genital tract. These processes only allow one male germ cell out of tens of millions to fuse with the oocyte. Sites of sperm selection in the female genital tract are the cervix, uterus, uterotubal junction, oviduct, cumulus oophorus and the zona pellucida. Newer strategies of sperm preparation are founded on: (i) morphological assessment by means of 'motile sperm organelle morphological examination (MSOME)'; (ii) electrical charge; and (iii) molecular binding characteristics of the sperm cell. Whereas separation methods based on electrical charge take advantage of the sperm's adherence to a test tube surface or separate in an electrophoresis, molecular binding techniques use Annexin V or hyaluronic acid (HA) as substrates. Techniques in this category are magnet-activated cell sorting, Annexin V-activated glass wool filtration, flow cytometry and picked spermatozoa for ICSI (PICSI) from HA-coated dishes and HA-containing media. Future developments may include Raman microspectrometry, confocal light absorption and scattering spectroscopic microscopy and polarization microscopy.
Figures
Similar articles
-
Improving ICSI: A review from the spermatozoon perspective.Syst Biol Reprod Med. 2016 Dec;62(6):359-371. doi: 10.1080/19396368.2016.1229365. Epub 2016 Sep 20. Syst Biol Reprod Med. 2016. PMID: 27646677 Review.
-
Effects of advanced selection methods on sperm quality and ART outcome.Minerva Ginecol. 2013 Oct;65(5):487-96. Minerva Ginecol. 2013. PMID: 24096286 Review.
-
Sperm processing for advanced reproductive technologies: Where are we today?Biotechnol Adv. 2016 Sep-Oct;34(5):578-587. doi: 10.1016/j.biotechadv.2016.01.007. Epub 2016 Feb 1. Biotechnol Adv. 2016. PMID: 26845061 Review.
-
The Behavior and Acrosomal Status of Mouse Spermatozoa In Vitro, and Within the Oviduct During Fertilization after Natural Mating.Biol Reprod. 2016 Sep;95(3):50. doi: 10.1095/biolreprod.116.140400. Epub 2016 Jul 14. Biol Reprod. 2016. PMID: 27417908
-
Could using the zona pellucida bound sperm for intracytoplasmic sperm injection (ICSI) enhance the outcome of ICSI?Asian J Androl. 2011 Mar;13(2):197-8. doi: 10.1038/aja.2010.179. Epub 2011 Jan 17. Asian J Androl. 2011. PMID: 21240292 Free PMC article. No abstract available.
Cited by
-
Effect of follicular fluid and platelet-activating factor on lactate dehydrogenase C expression in human asthenozoospermic samples.Iran J Med Sci. 2014 Jan;39(1):20-8. Iran J Med Sci. 2014. PMID: 24453390 Free PMC article.
-
Reduction of truncated Kit Expression in Men with Abnormal Semen Parameters, Globozoospermia and History of Low or Fertilization Failure.Cell J. 2019 Oct;21(3):314-321. doi: 10.22074/cellj.2019.6112. Epub 2019 Jun 15. Cell J. 2019. PMID: 31210438 Free PMC article.
-
Pronuclear formation by ICSI using chemically activated ovine oocytes and zona pellucida bound sperm.J Anim Sci Biotechnol. 2016 Nov 8;7:65. doi: 10.1186/s40104-016-0124-6. eCollection 2016. J Anim Sci Biotechnol. 2016. PMID: 27826442 Free PMC article.
-
Impact of the Z potential technique on reducing the sperm DNA fragmentation index, fertilization rate and embryo development.JBRA Assist Reprod. 2017 Dec 1;21(4):351-355. doi: 10.5935/1518-0557.20170055. JBRA Assist Reprod. 2017. PMID: 29072049 Free PMC article.
-
PICSI vs. MACS for abnormal sperm DNA fragmentation ICSI cases: a prospective randomized trial.J Assist Reprod Genet. 2020 Oct;37(10):2605-2613. doi: 10.1007/s10815-020-01913-4. Epub 2020 Aug 8. J Assist Reprod Genet. 2020. PMID: 32772268 Free PMC article.
References
-
- Williams M, Hill CJ, Scudamore I, Dunphy B, Cooke ID, et al. Sperm numbers and distribution within the human fallopian tube around ovulation. Hum Reprod. 1993;8:2019–26. - PubMed
-
- Eisenbach M, Giojalas LC. Sperm guidance in mammals—an unpaved road to the egg. Nat Rev Mol Cell Biol. 2006;7:276–85. - PubMed
-
- Henkel R.Sperm processing for IVFIn: Nagy ZP, Varghese A, Agarwal A. Practical Manual of In Vitro Fertilization Advanced Methods and Novel Devices Springer; 2012. In press.
-
- Franken DR, Henkel R.Sperm functional assaysIn: Tandulwadkar S, Mittal B, eds. Optimizing IUI Results—A Guide to Gynecologists New Delhi; Jaypee Brothers Medical Publishers (P) Ltd; 2010pp155–67.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

