Conventional and unconventional mechanisms for capping viral mRNA
- PMID: 22138959
- PMCID: PMC7097100
- DOI: 10.1038/nrmicro2675
Conventional and unconventional mechanisms for capping viral mRNA
Abstract
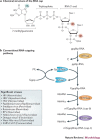
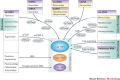
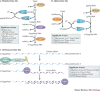
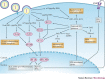
In the eukaryotic cell, capping of mRNA 5' ends is an essential structural modification that allows efficient mRNA translation, directs pre-mRNA splicing and mRNA export from the nucleus, limits mRNA degradation by cellular 5'-3' exonucleases and allows recognition of foreign RNAs (including viral transcripts) as 'non-self'. However, viruses have evolved mechanisms to protect their RNA 5' ends with either a covalently attached peptide or a cap moiety (7-methyl-Gppp, in which p is a phosphate group) that is indistinguishable from cellular mRNA cap structures. Viral RNA caps can be stolen from cellular mRNAs or synthesized using either a host- or virus-encoded capping apparatus, and these capping assemblies exhibit a wide diversity in organization, structure and mechanism. Here, we review the strategies used by viruses of eukaryotic cells to produce functional mRNA 5'-caps and escape innate immunity.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

