N-3-oxo-decanoyl-L-homoserine-lactone activates auxin-induced adventitious root formation via hydrogen peroxide- and nitric oxide-dependent cyclic GMP signaling in mung bean
- PMID: 22138973
- PMCID: PMC3271762
- DOI: 10.1104/pp.111.185769
N-3-oxo-decanoyl-L-homoserine-lactone activates auxin-induced adventitious root formation via hydrogen peroxide- and nitric oxide-dependent cyclic GMP signaling in mung bean
Abstract
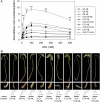
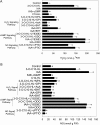
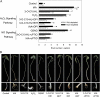
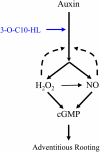
N-Acyl-homoserine-lactones (AHLs) are bacterial quorum-sensing signaling molecules that regulate population density. Recent evidence demonstrates their roles in plant defense responses and root development. Hydrogen peroxide (H(2)O(2)), nitric oxide (NO), and cyclic GMP (cGMP) are essential messengers that participate in various plant physiological processes, but how these messengers modulate the plant response to N-acyl-homoserine-lactone signals remains poorly understood. Here, we show that the N-3-oxo-decanoyl-homoserine-lactone (3-O-C10-HL), in contrast to its analog with an unsubstituted branch chain at the C3 position, efficiently stimulated the formation of adventitious roots and the expression of auxin-response genes in explants of mung bean (Vigna radiata) seedlings. This response was mimicked by the exogenous application of auxin, H(2)O(2), NO, or cGMP homologs but suppressed by treatment with scavengers or inhibitors of H(2)O(2), NO, or cGMP metabolism. The 3-O-C10-HL treatment enhanced auxin basipetal transport; this effect could be reversed by treatment with H(2)O(2) or NO scavengers but not by inhibitors of cGMP synthesis. Inhibiting 3-O-C10-HL-induced H(2)O(2) or NO accumulation impaired auxin- or 3-O-C10-HL-induced cGMP synthesis; however, blocking cGMP synthesis did not affect auxin- or 3-O-C10-HL-induced H(2)O(2) or NO generation. Additionally, cGMP partially rescued the inhibitory effect of H(2)O(2) or NO scavengers on 3-O-C10-HL-induced adventitious root development and auxin-response gene expression. These results suggest that 3-O-C10-HL, unlike its analog with an unmodified branch chain at the C3 position, can accelerate auxin-dependent adventitious root formation, possibly via H(2)O(2)- and NO-dependent cGMP signaling in mung bean seedlings.
Figures





References
-
- Bauer WD, Mathesius U. (2004) Plant responses to bacterial quorum sensing signals. Curr Opin Plant Biol 7: 429–433 - PubMed
-
- Campos-Cuevas JC, Pelagio-Flores R, Raya-González J, Méndez-Bravo A, Ortiz-Castro R, López-Bucio J. (2008) Tissue culture of Arabidopsis thaliana explants reveals a stimulatory effect of alkamides on adventitious root formation and nitric oxide accumulation. Plant Sci 174: 165–173
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Miscellaneous

