Improvement of the redox balance increases L-valine production by Corynebacterium glutamicum under oxygen deprivation conditions
- PMID: 22138982
- PMCID: PMC3264131
- DOI: 10.1128/AEM.07056-11
Improvement of the redox balance increases L-valine production by Corynebacterium glutamicum under oxygen deprivation conditions
Abstract
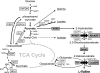
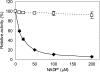
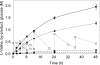
Production of L-valine under oxygen deprivation conditions by Corynebacterium glutamicum lacking the lactate dehydrogenase gene ldhA and overexpressing the L-valine biosynthesis genes ilvBNCDE was repressed. This was attributed to imbalanced cofactor production and consumption in the overall L-valine synthesis pathway: two moles of NADH was generated and two moles of NADPH was consumed per mole of L-valine produced from one mole of glucose. In order to solve this cofactor imbalance, the coenzyme requirement for L-valine synthesis was converted from NADPH to NADH via modification of acetohydroxy acid isomeroreductase encoded by ilvC and introduction of Lysinibacillus sphaericus leucine dehydrogenase in place of endogenous transaminase B, encoded by ilvE. The intracellular NADH/NAD(+) ratio significantly decreased, and glucose consumption and L-valine production drastically improved. Moreover, L-valine yield increased and succinate formation decreased concomitantly with the decreased intracellular redox state. These observations suggest that the intracellular NADH/NAD(+) ratio, i.e., reoxidation of NADH, is the primary rate-limiting factor for L-valine production under oxygen deprivation conditions. The L-valine productivity and yield were even better and by-products derived from pyruvate further decreased as a result of a feedback resistance-inducing mutation in the acetohydroxy acid synthase encoded by ilvBN. The resultant strain produced 1,470 mM L-valine after 24 h with a yield of 0.63 mol mol of glucose(-1), and the L-valine productivity reached 1,940 mM after 48 h.
Figures

References
-
- Ahn HJ, et al. 2003. Crystal structure of class I acetohydroxy acid isomeroreductase from Pseudomonas aeruginosa. J. Mol. Biol. 328:505–515 - PubMed
-
- Akashi K, Shibai H, Hirose Y. 1979. Effect of oxygen supply on l-phenylalanine, l-proline, l-glutamine and l-arginine fermentation. J. Ferment. Technol. 57:321–327
-
- Bartek T, et al. 2010. Importance of NADPH supply for improved l-valine formation in Corynebacterium glutamicum. Biotechnol. Prog. 26:361–371 - PubMed
-
- Blombach B, Schreiner ME, Bartek T, Oldiges M, Eikmanns BJ. 2008. Corynebacterium glutamicum tailored for high-yield l-valine production. Appl. Microbiol. Biotechnol. 79:471–479 - PubMed
-
- Bormann ER, Eikmanns BJ, Sahm H. 1992. Molecular analysis of the Corynebacterium glutamicum gdh gene encoding glutamate dehydrogenase. Mol. Microbiol. 6:317–326 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

