Suppression of Tumorigenicity-14, encoding matriptase, is a critical suppressor of colitis and colitis-associated colon carcinogenesis
- PMID: 22139080
- PMCID: PMC3299858
- DOI: 10.1038/onc.2011.545
Suppression of Tumorigenicity-14, encoding matriptase, is a critical suppressor of colitis and colitis-associated colon carcinogenesis
Abstract
Colitis-associated colorectal cancers are an etiologically distinct subgroup of colon cancers that occur in individuals suffering from inflammatory bowel disease and arise as a consequence of persistent exposure of hyperproliferative epithelial stem cells to an inflammatory microenvironment. An intrinsic defect in the intestinal epithelial barrier has been proposed to be one of several factors that contribute to the inappropriate immune response to the commensal microbiota that underlies inflammatory bowel disease. Matriptase is a membrane-anchored serine protease encoded by Suppression of Tumorigenicity-14 (ST14) that strengthens the intestinal epithelial barrier by promoting tight junction formation. Here, we show that intestinal epithelial-specific ablation of St14 in mice causes formation of colon adenocarcinoma with very early onset and high penetrance. Neoplastic progression is preceded by a chronic inflammation of the colon that resembles human inflammatory bowel disease and is promoted by the commensal microbiota. This study demonstrates that inflammation-associated colon carcinogenesis can be initiated and promoted solely by an intrinsic intestinal permeability barrier perturbation, establishes St14 as a critical tumor-suppressor gene in the mouse gastrointestinal tract and adds matriptase to the expanding list of pericellular proteases with tumor-suppressive functions.
Conflict of interest statement
The authors declare no competing financial interests in relation to the work described.
Figures
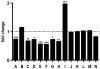



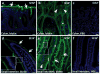



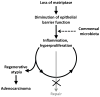
References
-
- Acuff HB, Sinnamon M, Fingleton B, Boone B, Levy SE, Chen X, et al. Analysis of host- and tumor-derived proteinases using a custom dual species microarray reveals a protective role for stromal matrix metalloproteinase-12 in non-small cell lung cancer. Cancer Res. 2006;66:7968–7975. - PubMed
-
- Balbin M, Fueyo A, Tester AM, Pendas AM, Pitiot AS, Astudillo A, et al. Loss of collagenase-2 confers increased skin tumor susceptibility to male mice. Nat Genet. 2003;35:252–257. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

