The human cadherin 11 is a pro-apoptotic tumor suppressor modulating cell stemness through Wnt/β-catenin signaling and silenced in common carcinomas
- PMID: 22139084
- PMCID: PMC3426851
- DOI: 10.1038/onc.2011.541
The human cadherin 11 is a pro-apoptotic tumor suppressor modulating cell stemness through Wnt/β-catenin signaling and silenced in common carcinomas
Abstract
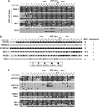
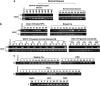
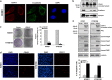
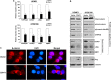
Genetic alterations of 16q21-q22, the locus of a 6-cadherin cluster, are frequently involved in multiple tumors, suggesting the presence of critical tumor suppressor genes (TSGs). Using 1 Mb array comparative genomic hybridization (aCGH), we refined a small hemizygous deletion (~1 Mb) at 16q21-22.1, which contains a single gene Cadherin-11 (CDH11, OB-cadherin). CDH11 was broadly expressed in human normal adult and fetal tissues, while its silencing and promoter CpG methylation were frequently detected in tumor cell lines, but not in immortalized normal epithelial cells. Aberrant methylation was also frequently detected in multiple primary tumors. CDH11 silencing could be reversed by pharmacologic or genetic demethylation, indicating an epigenetic mechanism. Ectopic expression of CDH11 strongly suppressed tumorigenecity and induced tumor cell apoptosis. Moreover, CDH11 was found to inhibit Wnt/β-catenin and AKT/Rho A signaling, as well as actin stress fiber formation, thus further inhibiting tumor cell migration and invasion. CDH11 also inhibited epithelial-to-mesenchymal transition and downregulated stem cell markers. Thus, our work identifies CDH11 as a functional tumor suppressor and an important antagonist of Wnt/β-catenin and AKT/Rho A signaling, with frequent epigenetic inactivation in common carcinomas.
Figures


Similar articles
-
WNT5A antagonizes WNT/β-catenin signaling and is frequently silenced by promoter CpG methylation in esophageal squamous cell carcinoma.Cancer Biol Ther. 2010 Sep 15;10(6):617-24. doi: 10.4161/cbt.10.6.12609. Epub 2010 Sep 7. Cancer Biol Ther. 2010. PMID: 20603606
-
Protocadherin20 Acts as a Tumor Suppressor Gene: Epigenetic Inactivation in Nasopharyngeal Carcinoma.J Cell Biochem. 2015 Aug;116(8):1766-75. doi: 10.1002/jcb.25135. J Cell Biochem. 2015. PMID: 25736877
-
Epigenetic identification of receptor tyrosine kinase-like orphan receptor 2 as a functional tumor suppressor inhibiting β-catenin and AKT signaling but frequently methylated in common carcinomas.Cell Mol Life Sci. 2014 Jun;71(11):2179-92. doi: 10.1007/s00018-013-1485-z. Epub 2013 Oct 25. Cell Mol Life Sci. 2014. PMID: 24158497 Free PMC article.
-
Gene of the month: E-cadherin (CDH1).J Clin Pathol. 2013 Nov;66(11):928-32. doi: 10.1136/jclinpath-2013-201768. Epub 2013 Aug 12. J Clin Pathol. 2013. PMID: 23940132 Review. No abstract available.
-
Epigenetic disruption of the WNT/beta-catenin signaling pathway in human cancers.Epigenetics. 2009 Jul 1;4(5):307-12. Epub 2009 Jul 25. Epigenetics. 2009. PMID: 19633433 Review.
Cited by
-
Beyond E-cadherin: roles of other cadherin superfamily members in cancer.Nat Rev Cancer. 2014 Feb;14(2):121-34. doi: 10.1038/nrc3647. Epub 2014 Jan 20. Nat Rev Cancer. 2014. PMID: 24442140 Review.
-
Phosphorylated Platelet-Derived Growth Factor Receptor-Positive Cells With Anti-apoptotic Properties Accumulate in the Synovium of Patients With Rheumatoid Arthritis.Front Immunol. 2019 Feb 15;10:241. doi: 10.3389/fimmu.2019.00241. eCollection 2019. Front Immunol. 2019. PMID: 30828336 Free PMC article.
-
Cadherin-11 Is Required for Neural Crest Specification and Survival.Front Physiol. 2020 Oct 30;11:563372. doi: 10.3389/fphys.2020.563372. eCollection 2020. Front Physiol. 2020. PMID: 33192560 Free PMC article.
-
Despicable role of epithelial-mesenchymal transition in breast cancer metastasis: Exhibiting de novo restorative regimens.Cancer Pathog Ther. 2024 Jan 12;3(1):30-47. doi: 10.1016/j.cpt.2024.01.001. eCollection 2025 Jan. Cancer Pathog Ther. 2024. PMID: 39872366 Free PMC article. Review.
-
Pathogenic variants in CDH11 impair cell adhesion and cause Teebi hypertelorism syndrome.Hum Genet. 2021 Jul;140(7):1061-1076. doi: 10.1007/s00439-021-02274-3. Epub 2021 Apr 3. Hum Genet. 2021. PMID: 33811546 Free PMC article.
References
-
- Andersson AM, Edvardsen K, Skakkebaek NE. Expression and localization of N- and E-cadherin in the human testis and epididymis. Int J Androl. 1994;17:174–180. - PubMed
-
- Andreeva AV, Kutuzov MA. Cadherin 13 in cancer. Genes Chromosomes Cancer. 2010;49:775–790. - PubMed
-
- Angst BD, Marcozzi C, Magee AI. The cadherin superfamily: diversity in form and function. J Cell Sci. 2001;114:629–641. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

