Culture of human mesenchymal stem cells at low oxygen tension improves growth and genetic stability by activating glycolysis
- PMID: 22139129
- PMCID: PMC3321628
- DOI: 10.1038/cdd.2011.172
Culture of human mesenchymal stem cells at low oxygen tension improves growth and genetic stability by activating glycolysis
Abstract
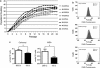
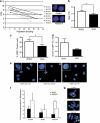
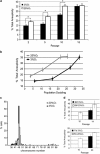
Expansion of human stem cells before cell therapy is typically performed at 20% O(2). Growth in these pro-oxidative conditions can lead to oxidative stress and genetic instability. Here, we demonstrate that culture of human mesenchymal stem cells at lower, physiological O(2) concentrations significantly increases lifespan, limiting oxidative stress, DNA damage, telomere shortening and chromosomal aberrations. Our gene expression and bioenergetic data strongly suggest that growth at reduced oxygen tensions favors a natural metabolic state of increased glycolysis and reduced oxidative phosphorylation. We propose that this balance is disturbed at 20% O(2), resulting in abnormally increased levels of oxidative stress. These observations indicate that bioenergetic pathways are intertwined with the control of lifespan and decisively influence the genetic stability of human primary stem cells. We conclude that stem cells for human therapy should be grown under low oxygen conditions to increase biosafety.
Figures


References
-
- Siegel G, Schafer R, Dazzi F. The immunosuppressive properties of mesenchymal stem cells. Transplantation. 2009;87 (9 Suppl:S45–S49. - PubMed
-
- Miyahara Y, Nagaya N, Kataoka M, Yanagawa B, Tanaka K, Hao H, et al. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat Med. 2006;12:459–465. - PubMed
-
- Vaupel P, Okunieff P, Neuringer LJ. Blood flow, tissue oxygenation, pH distribution, and energy metabolism of murine mammary adenocarcinomas during growth. Adv Exp Med Biol. 1989;248:835–845. - PubMed
-
- Fischer B, Bavister BD. Oxygen tension in the oviduct and uterus of rhesus monkeys, hamsters and rabbits. J Reprod Fertil. 1993;99:673–679. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

