Overexpression, purification, crystallization and preliminary crystallographic studies of a hyperthermophilic adenylosuccinate synthetase from Pyrococcus horikoshii OT3
- PMID: 22139164
- PMCID: PMC3232137
- DOI: 10.1107/S174430911104108X
Overexpression, purification, crystallization and preliminary crystallographic studies of a hyperthermophilic adenylosuccinate synthetase from Pyrococcus horikoshii OT3
Abstract
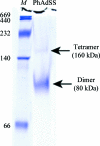
Adenylosuccinate synthetase (AdSS) is a ubiquitous enzyme that catalyzes the first committed step in the conversion of inosine monophosphate (IMP) to adenosine monophosphate (AMP) in the purine-biosynthetic pathway. Although AdSS from the vast majority of organisms is 430-457 amino acids in length, AdSS sequences isolated from thermophilic archaea are 90-120 amino acids shorter. In this study, crystallographic studies of a short AdSS sequence from Pyrococcus horikoshii OT3 (PhAdSS) were performed in order to reveal the unusual structure of AdSS from thermophilic archaea. Crystals of PhAdSS were obtained by the microbatch-under-oil method and X-ray diffraction data were collected to 2.50 Å resolution. The crystal belonged to the trigonal space group P3(2)12, with unit-cell parameters a = b = 57.2, c = 107.9 Å. There was one molecule per asymmetric unit, giving a Matthews coefficient of 2.17 Å(3) Da(-1) and an approximate solvent content of 43%. In contrast, the results of native polyacrylamide gel electrophoresis and analytical ultracentrifugation showed that the recombinant PhAdSS formed a dimer in solution.
Figures


References
-
- Bass, M. B., Fromm, H. J. & Rudolph, F. B. (1984). J. Biol. Chem. 259, 12330–12333. - PubMed
-
- Borza, T., Iancu, C. V., Pike, E., Honzatko, R. B. & Fromm, H. J. (2003). J. Biol. Chem. 278, 6673–6679. - PubMed
-
- Bouyoub, A., Barbier, G., Forterre, P. & Labedan, B. (1996). J. Mol. Biol. 261, 144–154. - PubMed
-
- Cann, I. K., Kanai, S., Toh, H. & Ishino, Y. (1998). Syst. Appl. Microbiol. 21, 478–486. - PubMed
-
- Eaazhisai, K., Jayalakshmi, R., Gayathri, P., Anand, R. P., Sumathy, K., Balaram, H. & Murthy, M. R. N. (2004). J. Mol. Biol. 335, 1251–1264. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

