An observational cohort study of extended dosing (once every 2 weeks or once monthly) regimens with darbepoetin alfa in patients with chronic kidney disease not on dialysis: the EXTEND study
- PMID: 22140136
- PMCID: PMC3363980
- DOI: 10.1093/ndt/gfr677
An observational cohort study of extended dosing (once every 2 weeks or once monthly) regimens with darbepoetin alfa in patients with chronic kidney disease not on dialysis: the EXTEND study
Abstract
Background: Darbepoetin alfa (DA) has been shown to be an effective treatment of anaemia in patients with chronic kidney disease (CKD) not on dialysis (NoD). EXTEND is an observational study assessing the effectiveness of DA administered once biweekly (Q2W) or monthly (QM) in a general CKD-NoD population.
Methods: Adult CKD-NoD patients starting DA Q2W/QM treatment in June 2006 or later were eligible. Retrospective and/or prospective data including haemoglobin levels and erythropoiesis-stimulating agent (ESA) dosing were collected for 6 months before and 12 months after DA initiation. Mean Hb levels were calculated every 3 months, and ESA dose was converted to a geometric mean weekly DA equivalent dose and summarized monthly.
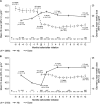
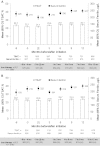
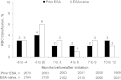
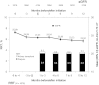
Results: Data from 4278 patients showed that patients receiving ESA treatment before DA Q2W/QM initiation had a mean (95% confidence interval) Hb level of 11.9 g/dL (11.8-12.0 g/dL) at initiation and 11.6 g/dL (11.6-11.7 g/dL) at Months 10-12, with mean ESA dose of 22 μg/week (21-23 μg/week) prior to initiation, 16 μg/week (15-16 μg/week) at initiation and 16 μg/week (15-16 μg/week) at Month 12. In ESA-naive patients, Hb levels increased from 10.3 g/dL (10.2-10.3 g/dL) at initiation to 11.7 g/dL at Months 4-6 and were maintained at a mean level of 11.7 g/dL (11.7-11.8 g/dL) at Months 10-12, with mean ESA dose of 16 μg/week (16-17 μg/week) at initiation and 16 μg/week (16-17 μg/week) at Month 12. In the 85% of patients receiving DA at extended intervals (Q2W or less frequently) at Month 12, 12 patients (0.3%) experienced DA-related adverse reactions.
Conclusion: DA Q2W/QM was an effective treatment of anaemia in the general CKD-NoD patient population and a dose increase was not required in patients switching from a previous ESA regimen.
Figures
Similar articles
-
Outcomes in patients with chronic kidney disease not on dialysis receiving extended dosing regimens of darbepoetin alfa: long-term results of the EXTEND observational cohort study.Nephrol Dial Transplant. 2016 Dec;31(12):2073-2085. doi: 10.1093/ndt/gfw047. Epub 2016 Apr 15. Nephrol Dial Transplant. 2016. PMID: 27190334 Free PMC article.
-
Darbepoetin alfa once monthly corrects anaemia in patients with chronic kidney disease not on dialysis.Nephrology (Carlton). 2014 May;19(5):266-74. doi: 10.1111/nep.12214. Nephrology (Carlton). 2014. PMID: 24506498 Clinical Trial.
-
Effect of darbepoetin alfa administered once monthly on maintaining hemoglobin levels in older patients with chronic kidney disease.Am J Geriatr Pharmacother. 2008 Jun;6(2):49-60. doi: 10.1016/j.amjopharm.2008.05.002. Am J Geriatr Pharmacother. 2008. PMID: 18675764 Clinical Trial.
-
Darbepoetin alfa: a novel erythropoiesis-stimulating protein.Drugs Today (Barc). 2003 Jul;39(7):477-95. doi: 10.1358/dot.2003.39.7.799441. Drugs Today (Barc). 2003. PMID: 12973399 Review.
-
Dosing patterns, drug costs, and hematologic outcome in anemic patients with chronic kidney disease switching from darbepoetin alfa to epoetin alfa.Curr Med Res Opin. 2007 Aug;23(8):1931-7. doi: 10.1185/030079907X210705. Curr Med Res Opin. 2007. PMID: 17624232 Review.
Cited by
-
Outcomes in patients with chronic kidney disease not on dialysis receiving extended dosing regimens of darbepoetin alfa: long-term results of the EXTEND observational cohort study.Nephrol Dial Transplant. 2016 Dec;31(12):2073-2085. doi: 10.1093/ndt/gfw047. Epub 2016 Apr 15. Nephrol Dial Transplant. 2016. PMID: 27190334 Free PMC article.
-
Differentiating factors between erythropoiesis-stimulating agents: an update to selection for anaemia of chronic kidney disease.Drugs. 2013 Feb;73(2):117-30. doi: 10.1007/s40265-012-0002-2. Drugs. 2013. PMID: 23338536 Review.
References
-
- Cody J, Daly C, Campbell M, et al. Recombinant human erythropoietin for chronic renal failure anaemia in pre-dialysis patients. Cochrane Database Syst Rev. 2005;((3):) CD003266. - PubMed
-
- Toto RD, Pichette V, Navarro J, et al. Darbepoetin alfa effectively treats anemia in patients with chronic kidney disease with de novo every-other-week administration. Am J Nephrol. 2004;24:453–460. - PubMed
-
- Suranyi MG, Lindberg JS, Navarro J, et al. Treatment of anemia with darbepoetin alfa administered de novo once every other week in chronic kidney disease. Am J Nephrol. 2003;23:106–111. - PubMed
-
- Agarwal AK, Silver MR, Reed JE, et al. An open-label study of darbepoetin alfa administered once monthly for the maintenance of haemoglobin concentrations in patients with chronic kidney disease not receiving dialysis. J Intern Med. 2006;260:577–585. - PubMed
-
- Disney A, Jersey PD, Kirkland G, et al. Darbepoetin alfa administered monthly maintains haemoglobin concentrations in patients with chronic kidney disease not receiving dialysis: a multicentre, open-label, Australian study. Nephrology (Carlton) 2007;12:95–101. - PubMed

