Transcription factor Efg1 shows a haploinsufficiency phenotype in modulating the cell wall architecture and immunogenicity of Candida albicans
- PMID: 22140230
- PMCID: PMC3272906
- DOI: 10.1128/EC.05206-11
Transcription factor Efg1 shows a haploinsufficiency phenotype in modulating the cell wall architecture and immunogenicity of Candida albicans
Abstract
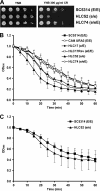
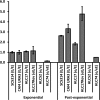
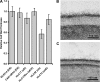
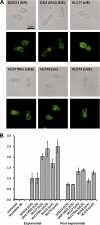
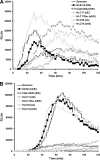
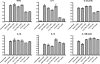
The Candida albicans transcription factor Efg1 is known to be involved in many different cellular processes, including morphogenesis, general metabolism, and virulence. Here we show that besides its manifold roles, Efg1 also has a prominent effect on cell wall structure and composition, strongly affecting the structural glucan part. Deletion of only one allele of EFG1 already results in severe phenotypes for cell wall biogenesis, comparable to those with deletion of both alleles, indicative of a severe haploinsufficiency for EFG1. The observed defects in structural setup of the cell wall, together with previously reported alterations in expression of cell surface proteins, result in altered immunogenic properties of strains with compromised Efg1 function. This is shown by interaction studies with macrophages and primary dendritic cells. The structural changes in the cell wall carbohydrate meshwork presented here, together with the manifold changes in cell wall protein composition and metabolism reported in other studies, contribute to the altered immune response mounted by innate immune cells and to the altered virulence phenotypes observed for strains lacking EFG1.
Figures
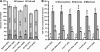
Similar articles
-
Efg1 Controls caspofungin-induced cell aggregation of Candida albicans through the adhesin Als1.Eukaryot Cell. 2011 Dec;10(12):1694-704. doi: 10.1128/EC.05187-11. Epub 2011 Oct 28. Eukaryot Cell. 2011. PMID: 22037180 Free PMC article.
-
EFG1 is a major regulator of cell wall dynamics in Candida albicans as revealed by DNA microarrays.Mol Microbiol. 2003 Jan;47(1):89-102. doi: 10.1046/j.1365-2958.2003.03300.x. Mol Microbiol. 2003. PMID: 12492856
-
Efg1 and Cas5 Orchestrate Cell Wall Damage Response to Caspofungin in Candida albicans.Antimicrob Agents Chemother. 2021 Jan 20;65(2):e01584-20. doi: 10.1128/AAC.01584-20. Print 2021 Jan 20. Antimicrob Agents Chemother. 2021. PMID: 33168610 Free PMC article.
-
EFG1, Everyone's Favorite Gene in Candida albicans: A Comprehensive Literature Review.Front Cell Infect Microbiol. 2022 Mar 22;12:855229. doi: 10.3389/fcimb.2022.855229. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35392604 Free PMC article. Review.
-
Histone deacetylase-mediated morphological transition in Candida albicans.J Microbiol. 2015 Dec;53(12):805-11. doi: 10.1007/s12275-015-5488-3. Epub 2015 Dec 2. J Microbiol. 2015. PMID: 26626350 Review.
Cited by
-
Virulence and biofilms as promising targets in developing antipathogenic drugs against candidiasis.Future Sci OA. 2020 Feb 3;6(2):FSO440. doi: 10.2144/fsoa-2019-0027. Future Sci OA. 2020. PMID: 32025329 Free PMC article. Review.
-
Potential Targets for Antifungal Drug Discovery Based on Growth and Virulence in Candida albicans.Antimicrob Agents Chemother. 2015 Oct;59(10):5885-91. doi: 10.1128/AAC.00726-15. Epub 2015 Jul 20. Antimicrob Agents Chemother. 2015. PMID: 26195510 Free PMC article. Review.
-
Activation and alliance of regulatory pathways in C. albicans during mammalian infection.PLoS Biol. 2015 Feb 18;13(2):e1002076. doi: 10.1371/journal.pbio.1002076. eCollection 2015 Feb. PLoS Biol. 2015. PMID: 25693184 Free PMC article.
-
RNA sequencing revealed novel actors of the acquisition of drug resistance in Candida albicans.BMC Genomics. 2012 Aug 16;13:396. doi: 10.1186/1471-2164-13-396. BMC Genomics. 2012. PMID: 22897889 Free PMC article.
-
Novel insight into neutrophil immune responses by dry mass determination of Candida albicans morphotypes.PLoS One. 2013 Oct 30;8(10):e77993. doi: 10.1371/journal.pone.0077993. eCollection 2013. PLoS One. 2013. PMID: 24205058 Free PMC article.
References
-
- Bourgeois C, Majer O, Frohner IE, Tierney L, Kuchler K. 2010. Fungal attacks on mammalian hosts: pathogen elimination requires sensing and tasting. Curr. Opin. Microbiol. 13:401–408 - PubMed
-
- Care RS, Trevethick J, Binley KM, Sudbery PE. 1999. The MET3 promoter: a new tool for Candida albicans molecular genetics. Mol. Microbiol. 34:792–798 - PubMed
-
- Chamilos G, et al. 2006. Drosophila melanogaster as a facile model for large-scale studies of virulence mechanisms and antifungal drug efficacy in Candida species. J. Infect. Dis. 193:1014–1022 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

