A genome-wide association study of men with symptoms of testicular dysgenesis syndrome and its network biology interpretation
- PMID: 22140272
- PMCID: PMC3284313
- DOI: 10.1136/jmedgenet-2011-100174
A genome-wide association study of men with symptoms of testicular dysgenesis syndrome and its network biology interpretation
Abstract
Background: Testicular dysgenesis syndrome (TDS) is a common disease that links testicular germ cell cancer, cryptorchidism and some cases of hypospadias and male infertility with impaired development of the testis. The incidence of these disorders has increased over the last few decades, and testicular cancer now affects 1% of the Danish and Norwegian male population.
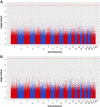
Methods: To identify genetic variants that span the four TDS phenotypes, the authors performed a genome-wide association study (GWAS) using Affymetrix Human SNP Array 6.0 to screen 488 patients with symptoms of TDS and 439 selected controls with excellent reproductive health. Furthermore, they developed a novel integrative method that combines GWAS data with other TDS-relevant data types and identified additional TDS markers. The most significant findings were replicated in an independent cohort of 671 Nordic men.
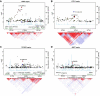
Results: Markers located in the region of TGFBR3 and BMP7 showed association with all TDS phenotypes in both the discovery and replication cohorts. An immunohistochemistry investigation confirmed the presence of transforming growth factor β receptor type III (TGFBR3) in peritubular and Leydig cells, in both fetal and adult testis. Single-nucleotide polymorphisms in the KITLG gene showed significant associations, but only with testicular cancer.
Conclusions: The association of single-nucleotide polymorphisms in the TGFBR3 and BMP7 genes, which belong to the transforming growth factor β signalling pathway, suggests a role for this pathway in the pathogenesis of TDS. Integrating data from multiple layers can highlight findings in GWAS that are biologically relevant despite having border significance at currently accepted statistical levels.
Conflict of interest statement
Figures


References
-
- Schnack TH, Poulsen G, Myrup C, Wohlfahrt J, Melbye M. Familial coaggregation of cryptorchidism, hypospadias, and testicular germ cell cancer: a nationwide cohort study. J Natl Cancer Inst 2010;102:187–92 - PubMed
-
- Skakkebaek NE, Rajpert-De Meyts E, Main KM. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum Reprod 2001;16:972–8 - PubMed
-
- Purdue MP, Devesa SS, Sigurdson AJ, McGlynn KA. International patterns and trends in testis cancer incidence. Int J Cancer 2005;115:822–7 - PubMed
-
- Engholm G, Ferlay J, Christensen N, Bray F, Gjerstorff ML, Klint A, Køtlum JE, Olafsdóttir E, Pukkala E, Storm HH. NORDCAN–a Nordic tool for cancer information, planning, quality control and research. Acta Oncol 2010;49:725–36 - PubMed
-
- Asklund C, Jensen TK, Main KM, Sobotka T, Skakkebaek NE, Jørgensen N. Semen quality, reproductive hormones and fertility of men operated for hypospadias. Int J Androl 2010;33:80–7 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
