Total syntheses of isonaamine C and isonaamidine E
- PMID: 22140280
- PMCID: PMC3225899
- DOI: 10.1016/j.tetlet.2011.08.030
Total syntheses of isonaamine C and isonaamidine E
Abstract
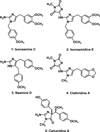
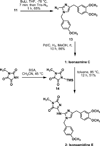
The total syntheses of two alkaloids isolated from a marine sponge of the Leucetta sp. have been accomplished in 6 and 7 steps starting from a 4,5-diiodoimidazole derivative. Grignard mediated halogen-metal exchange was used to install the benzyl side chain. C2 substitution was accomplished via lithiation followed by quenching with trisyl azide which provided isonaamine C after hydrogenation. Isonaamidine E was then prepared from isonaamine C via introduction of the hydantoin ring by reaction with an activated parabanic acid.
Figures
References
-
- Sullivan JD, Giles RL, Looper RE. Curr Bioact Comp. 2009;5:39–78.
-
- Koswatta PB, Lovely CJ. Nat Prod Rep. 2011;28:511–528. - PubMed
-
- Ermolat'ev DS, Alifanov VL, Rybakov VB, Babaev EV, Van der Eycken EV. Synthesis. 2008:2083–2088.
-
- Gross H, Kehraus S, Koenig GM, Woerheide G, Wright AD. J Nat Prod. 2002;65:1190–1193. - PubMed
-
-
The nomenclature for the natural products in this manuscript follows that of the original isolation paper (see ref 4).
-
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous