Ubiquitin conjugation of hepatitis B virus core antigen DNA vaccine leads to enhanced cell-mediated immune response in BALB/c mice
- PMID: 22140385
- PMCID: PMC3227483
- DOI: 10.5812/kowsar.1735143x.689
Ubiquitin conjugation of hepatitis B virus core antigen DNA vaccine leads to enhanced cell-mediated immune response in BALB/c mice
Abstract
Background: Nearly 350 million persons worldwide are chronically infected with hepatitis B virus (HBV). Ubiquitin (Ub) is a highly conserved small regulatory protein, ubiquitous in eukaryotes, that usually serves as a signal for the target protein that is recognised and degraded in proteasomes . The Ub-mediated processing of antigens is rapid and efficient and stimulates cell-mediated immune responses. Accordingly, Ub-mediated processing of antigens has been widely used in chronic-infection and cancer studies to improve immune response.
Objectives: Many clinical trials have shown that DNA vaccine potency needs to be greatly enhanced. Here, we report a new strategy for designing an HBV DNA vaccine using the ubiquitin (Ub) sequence. The aim of this study was to investigate a novel DNA vaccination, based on the expression of HBV core antigen (HBcAg), fused to Ub to enhance DNA vaccine potency.
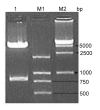
Materials and methods: Mouse ubiquitin fused to the HBcAg gene and cloned into the eukaryotic vector pcDNA3.1 (-). BALB/c mice were immunized with recombinant pUb-HBcAg or pHBcAg DNA vaccine. Lymphocyte proliferation assay, intracellular IFN-γ assay, CTL cytotoxicity assay, and antibody assay were performed to analyze the cellular and humoral immune responses to our DNA constructs.
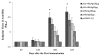
Results: HBcAg was expressed effectively in the COS-7 cells that were transiently transfected with pUb-HBcAg. Strong anti-HBc IgG responses were elicited in mice that were immunized with pUb-HBcAg. The endpoint titers of anti-HBc peaked at 1:656100 on the 42nd day after the third immunization. pUb-HBcAg stimulated greater lymphocyte proliferation and induced higher levels of IL-2 and IFN-γ and a greater percentage of HBcAg-specific CD8+ T cells in mice than pHBcAg. In the CTL assay, the specific lysis rate reached 56.5% at an effector:target ratio of 50:1 in mice that were immunized with pUb-HBcAg.
Conclusions: pUb-HBcAg elicits specific anti-HBc responses and induces HBc-specific CTL responses in immunized BALB/c mice. Our results imply that Ub can be used as a molecular adjuvant that enhances the potency of DNA vaccines.
Keywords: DNA vaccine; Hepatitis B core antigen; Ubiquitin.
Figures

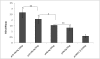
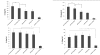

Similar articles
-
Lentiviral vector encoding ubiquitinated hepatitis B core antigen induces potent cellular immune responses and therapeutic immunity in HBV transgenic mice.Immunobiology. 2016 Jul;221(7):813-21. doi: 10.1016/j.imbio.2016.01.015. Epub 2016 Feb 2. Immunobiology. 2016. PMID: 26874581
-
Ubiquitin-hepatitis B core antigen-cytoplasmic transduction peptide enhances HBV-specific humoral and CTL immune responses in vivo.Int Immunopharmacol. 2014 Nov;23(1):1-7. doi: 10.1016/j.intimp.2014.08.006. Epub 2014 Aug 15. Int Immunopharmacol. 2014. PMID: 25135878
-
Novel DNA vaccine based on hepatitis B virus core gene induces specific immune responses in Balb/c mice.World J Gastroenterol. 2005 Aug 7;11(29):4583-6. doi: 10.3748/wjg.v11.i29.4583. World J Gastroenterol. 2005. PMID: 16052693 Free PMC article.
-
Hybrid hepatitis B virus core antigen as a vaccine carrier moiety: I. presentation of foreign epitopes.J Biotechnol. 1996 Jan 26;44(1-3):91-6. doi: 10.1016/0168-1656(95)00118-2. J Biotechnol. 1996. PMID: 8717391 Review.
-
Hybrid hepatitis B virus core antigen as a vaccine carrier moiety. II. Expression in avirulent Salmonella spp. for mucosal immunization.Adv Exp Med Biol. 1996;397:15-21. Adv Exp Med Biol. 1996. PMID: 8718577 Review.
Cited by
-
The future of human DNA vaccines.J Biotechnol. 2012 Dec 31;162(2-3):171-82. doi: 10.1016/j.jbiotec.2012.08.012. Epub 2012 Sep 7. J Biotechnol. 2012. PMID: 22981627 Free PMC article. Review.
-
DNA Vaccine Targeting Gonadotropin-Releasing Hormone Receptor and Its Application in Animal Contraception.Mol Biotechnol. 2019 Feb;61(2):73-83. doi: 10.1007/s12033-018-0137-9. Mol Biotechnol. 2019. PMID: 30448908
-
Rhoptry antigens as Toxoplasma gondii vaccine target.Clin Exp Vaccine Res. 2019 Jan;8(1):4-26. doi: 10.7774/cevr.2019.8.1.4. Epub 2019 Jan 31. Clin Exp Vaccine Res. 2019. PMID: 30775347 Free PMC article. Review.
-
Molecular identification of hepatitis B virus genotypes/subgenotypes: revised classification hurdles and updated resolutions.World J Gastroenterol. 2014 Jun 21;20(23):7152-68. doi: 10.3748/wjg.v20.i23.7152. World J Gastroenterol. 2014. PMID: 24966586 Free PMC article. Review.
-
New loci associated with chronic hepatitis B virus infection in Han Chinese.Nat Genet. 2013 Dec;45(12):1499-503. doi: 10.1038/ng.2809. Epub 2013 Oct 27. Nat Genet. 2013. PMID: 24162738
References
-
- Te HS, Jensen DM. Epidemiology of hepatitis B and C viruses: a global overview. Clin Liver Dis. 2010;14(1):1–21, vii. - PubMed
-
- Schneider J, Gilbert SC, Blanchard TJ, Hanke T, Robson KJ, Hannan CM, Becker M, Sinden R, Smith GL, Hill AV. Enhanced immunogenicity for CD8+ T cell induction and complete protective efficacy of malaria DNA vaccination by boosting with modified vaccinia virus Ankara. Nat Med. 1998;4(4):397–402. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
