Understanding the mechanism of insulin and insulin-like growth factor (IGF) receptor activation by IGF-II
- PMID: 22140443
- PMCID: PMC3227035
- DOI: 10.1371/journal.pone.0027488
Understanding the mechanism of insulin and insulin-like growth factor (IGF) receptor activation by IGF-II
Abstract
Background: Insulin-like growth factor-II (IGF-II) promotes cell proliferation and survival and plays an important role in normal fetal development and placental function. IGF-II binds both the insulin-like growth factor receptor (IGF-1R) and insulin receptor isoform A (IR-A) with high affinity. Interestingly both IGF-II and the IR-A are often upregulated in cancer and IGF-II acts via both receptors to promote cancer proliferation. There is relatively little known about the mechanism of ligand induced activation of the insulin (IR) and IGF-1R. The recently solved IR structure reveals a folded over dimer with two potential ligand binding pockets arising from residues on each receptor half. Site-directed mutagenesis has mapped receptor residues important for ligand binding to two separate sites within the ligand binding pocket and we have recently shown that the IGFs have two separate binding surfaces which interact with the receptor sites 1 and 2.
Methodology/principal findings: In this study we describe a series of partial IGF-1R and IR agonists generated by mutating Glu12 of IGF-II. By comparing receptor binding affinities, abilities to induce negative cooperativity and potencies in receptor activation, we provide evidence that residue Glu12 bridges the two receptor halves leading to receptor activation.
Conclusions/significance: This study provides novel insight into the mechanism of receptor binding and activation by IGF-II, which may be important for the future development of inhibitors of its action for the treatment of cancer.
Conflict of interest statement
Figures


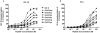
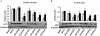
References
-
- Renehan AG, Brennan BM. Acromegaly, growth hormone and cancer risk. Best Pract Res Clin Endocrinol Metab. 2008;22:639–657. - PubMed
-
- Walenkamp MJ, Wit JM. Genetic disorders in the growth hormone - insulin-like growth factor-I axis. Horm Res. 2006;66:221–230. - PubMed
-
- Gicquel C, Le Bouc Y. Hormonal regulation of fetal growth. Horm Res. 2006;3:28–33. - PubMed
-
- Denley A, Wallace JC, Cosgrove LJ, Forbes BE. The insulin receptor isoform exon 11- (IR-A) in cancer and other diseases: a review. Horm Metab Res. 2003;35:778–785. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

