Raloxifene and desmethylarzoxifene block estrogen-induced malignant transformation of human breast epithelial cells
- PMID: 22140478
- PMCID: PMC3226622
- DOI: 10.1371/journal.pone.0027876
Raloxifene and desmethylarzoxifene block estrogen-induced malignant transformation of human breast epithelial cells
Abstract
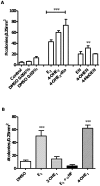
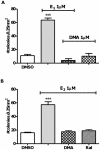
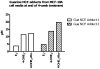
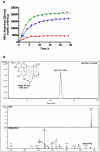
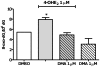
There is association between exposure to estrogens and the development and progression of hormone-dependent gynecological cancers. Chemical carcinogenesis by catechol estrogens derived from oxidative metabolism is thought to contribute to breast cancer, yet exact mechanisms remain elusive. Malignant transformation was studied in MCF-10A human mammary epithelial cells, since estrogens are not proliferative in this cell line. The human and equine estrogen components of estrogen replacement therapy (ERT) and their catechol metabolites were studied, along with the influence of co-administration of selective estrogen receptor modulators (SERMs), raloxifene and desmethyl-arzoxifene (DMA), and histone deacetylase inhibitors. Transformation was induced by human estrogens, and selectively by the 4-OH catechol metabolite, and to a lesser extent by an equine estrogen metabolite. The observed estrogen-induced upregulation of CYP450 1B1 in estrogen receptor negative MCF-10A cells, was compatible with a causal role for 4-OH catechol estrogens, as was attenuated transformation by CYP450 inhibitors. Estrogen-induced malignant transformation was blocked by SERMs correlating with a reduction in formation of nucleobase catechol estrogen (NCE) adducts and formation of 8-oxo-dG. NCE adducts can be formed consequent to DNA abasic site formation, but NCE adducts were also observed on incubation of estrogen quinones with free nucleotides. These results suggest that NCE adducts may be a biomarker for cellular electrophilic stress, which together with 8-oxo-dG as a biomarker of oxidative stress correlate with malignant transformation induced by estrogen oxidative metabolites. The observed attenuation of transformation by SERMs correlated with these biomarkers and may also be of clinical significance in breast cancer chemoprevention.
Conflict of interest statement
Figures



Similar articles
-
SERMs attenuate estrogen-induced malignant transformation of human mammary epithelial cells by upregulating detoxification of oxidative metabolites.Cancer Prev Res (Phila). 2014 May;7(5):505-15. doi: 10.1158/1940-6207.CAPR-13-0296. Epub 2014 Mar 5. Cancer Prev Res (Phila). 2014. PMID: 24598415 Free PMC article.
-
Redox cycling of catechol estrogens generating apurinic/apyrimidinic sites and 8-oxo-deoxyguanosine via reactive oxygen species differentiates equine and human estrogens.Chem Res Toxicol. 2010 Aug 16;23(8):1365-73. doi: 10.1021/tx1001282. Chem Res Toxicol. 2010. PMID: 20509668 Free PMC article.
-
Structural modulation of reactivity/activity in design of improved benzothiophene selective estrogen receptor modulators: induction of chemopreventive mechanisms.Mol Cancer Ther. 2007 Sep;6(9):2418-28. doi: 10.1158/1535-7163.MCT-07-0268. Mol Cancer Ther. 2007. PMID: 17876041
-
Mechanisms of estrogen carcinogenesis: The role of E2/E1-quinone metabolites suggests new approaches to preventive intervention--A review.Steroids. 2015 Jul;99(Pt A):56-60. doi: 10.1016/j.steroids.2014.08.006. Epub 2014 Aug 24. Steroids. 2015. PMID: 25159108 Free PMC article. Review.
-
Potential mechanisms of estrogen quinone carcinogenesis.Chem Res Toxicol. 2008 Jan;21(1):93-101. doi: 10.1021/tx700191p. Epub 2007 Dec 4. Chem Res Toxicol. 2008. PMID: 18052105 Free PMC article. Review.
Cited by
-
Effects of ospemifene on the female reproductive and urinary tracts: translation from preclinical models into clinical evidence.Menopause. 2015 Jul;22(7):786-96. doi: 10.1097/GME.0000000000000365. Menopause. 2015. PMID: 25423325 Free PMC article. Review.
-
SERMs attenuate estrogen-induced malignant transformation of human mammary epithelial cells by upregulating detoxification of oxidative metabolites.Cancer Prev Res (Phila). 2014 May;7(5):505-15. doi: 10.1158/1940-6207.CAPR-13-0296. Epub 2014 Mar 5. Cancer Prev Res (Phila). 2014. PMID: 24598415 Free PMC article.
-
Design and Synthesis of Basic Selective Estrogen Receptor Degraders for Endocrine Therapy Resistant Breast Cancer.J Med Chem. 2019 Dec 26;62(24):11301-11323. doi: 10.1021/acs.jmedchem.9b01580. Epub 2019 Dec 10. J Med Chem. 2019. PMID: 31746603 Free PMC article.
-
Selective Human Estrogen Receptor Partial Agonists (ShERPAs) for Tamoxifen-Resistant Breast Cancer.J Med Chem. 2016 Jan 14;59(1):219-237. doi: 10.1021/acs.jmedchem.5b01276. Epub 2015 Dec 30. J Med Chem. 2016. PMID: 26681208 Free PMC article.
-
Estrogen Drives Cellular Transformation and Mutagenesis in Cells Expressing the Breast Cancer-Associated R438W DNA Polymerase Lambda Protein.Mol Cancer Res. 2016 Nov;14(11):1068-1077. doi: 10.1158/1541-7786.MCR-16-0209. Epub 2016 Sep 12. Mol Cancer Res. 2016. PMID: 27621267 Free PMC article.
References
-
- Russo J, Hasan Lareef M, Balogh G, Guo S, et al. Estrogen and its metabolites are carcinogenic agents in human breast epithelial cells. J Steroid Biochem Mol Biol. 2003;87:1–25. - PubMed
-
- Liehr JG. Is estradiol a genotoxic mutagenic carcinogen? Endocr Rev. 2000;21:40–54. - PubMed
-
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. - PubMed
-
- Beral V. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;362:419–427. - PubMed
-
- Ravdin PM, Cronin KA, Howlader N, Berg CD, Chlebowski RT, et al. The decrease in breast-cancer incidence in 2003 in the United States. N Engl J Med. 2007;356:1670–1674. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

