A flavonoid, luteolin, cripples HIV-1 by abrogation of tat function
- PMID: 22140483
- PMCID: PMC3227592
- DOI: 10.1371/journal.pone.0027915
A flavonoid, luteolin, cripples HIV-1 by abrogation of tat function
Abstract
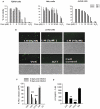
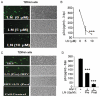
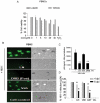
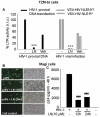
Despite the effectiveness of combination antiretroviral treatment (cART) against HIV-1, evidence indicates that residual infection persists in different cell types. Intensification of cART does not decrease the residual viral load or immune activation. cART restricts the synthesis of infectious virus but does not curtail HIV-1 transcription and translation from either the integrated or unintegrated viral genomes in infected cells. All treated patients with full viral suppression actually have low-level viremia. More than 60% of treated individuals also develop minor HIV-1 -associated neurocognitive deficits (HAND) due to residual virus and immune activation. Thus, new therapeutic agents are needed to curtail HIV-1 transcription and residual virus. In this study, luteolin, a dietary supplement, profoundly reduced HIV-1 infection in reporter cells and primary lymphocytes. HIV-1inhibition by luteolin was independent of viral entry, as shown by the fact that wild-type and VSV-pseudotyped HIV-1 infections were similarly inhibited. Luteolin was unable to inhibit viral reverse transcription. Luteolin had antiviral activity in a latent HIV-1 reactivation model and effectively ablated both clade-B- and -C -Tat-driven LTR transactivation in reporter assays but had no effect on Tat expression and its sub-cellular localization. We conclude that luteolin confers anti-HIV-1 activity at the Tat functional level. Given its biosafety profile and ability to cross the blood-brain barrier, luteolin may serve as a base flavonoid to develop potent anti-HIV-1 derivatives to complement cART.
Conflict of interest statement
Figures




Similar articles
-
Maca (Lepidium meyenii Walp.) inhibits HIV-1 infection through the activity of thiadiazole alkaloids in viral integration.J Ethnopharmacol. 2024 Dec 5;335:118613. doi: 10.1016/j.jep.2024.118613. Epub 2024 Jul 23. J Ethnopharmacol. 2024. PMID: 39047879
-
Inhibition of both HIV-1 reverse transcription and gene expression by a cyclic peptide that binds the Tat-transactivating response element (TAR) RNA.PLoS Pathog. 2011 May;7(5):e1002038. doi: 10.1371/journal.ppat.1002038. Epub 2011 May 19. PLoS Pathog. 2011. PMID: 21625572 Free PMC article.
-
Shutdown of HIV-1 Transcription in T Cells by Nullbasic, a Mutant Tat Protein.mBio. 2016 Jul 5;7(4):e00518-16. doi: 10.1128/mBio.00518-16. mBio. 2016. PMID: 27381288 Free PMC article.
-
Tat-Based Therapies as an Adjuvant for an HIV-1 Functional Cure.Viruses. 2020 Apr 8;12(4):415. doi: 10.3390/v12040415. Viruses. 2020. PMID: 32276443 Free PMC article. Review.
-
Recent advances in the identification of Tat-mediated transactivation inhibitors: progressing toward a functional cure of HIV.Future Med Chem. 2016;8(4):421-42. doi: 10.4155/fmc.16.3. Epub 2016 Mar 2. Future Med Chem. 2016. PMID: 26933891 Review.
Cited by
-
A comprehensive perspective of traditional Arabic or Islamic medicinal plants as an adjuvant therapy against COVID-19.Saudi J Biol Sci. 2023 Mar;30(3):103561. doi: 10.1016/j.sjbs.2023.103561. Epub 2023 Jan 13. Saudi J Biol Sci. 2023. PMID: 36684115 Free PMC article. Review.
-
Identification of luteolin as enterovirus 71 and coxsackievirus A16 inhibitors through reporter viruses and cell viability-based screening.Viruses. 2014 Jul 17;6(7):2778-95. doi: 10.3390/v6072778. Viruses. 2014. PMID: 25036464 Free PMC article.
-
EBV reactivation as a target of luteolin to repress NPC tumorigenesis.Oncotarget. 2016 Apr 5;7(14):18999-9017. doi: 10.18632/oncotarget.7967. Oncotarget. 2016. PMID: 26967558 Free PMC article.
-
Boosting immunity: synergistic antiviral effects of luteolin, vitamin C, magnesium and zinc against SARS-CoV-2 3CLpro.Biosci Rep. 2024 Aug 28;44(8):BSR20240617. doi: 10.1042/BSR20240617. Biosci Rep. 2024. PMID: 39045772 Free PMC article.
-
Pedilanthus tithymaloides Inhibits HSV Infection by Modulating NF-κB Signaling.PLoS One. 2015 Sep 25;10(9):e0139338. doi: 10.1371/journal.pone.0139338. eCollection 2015. PLoS One. 2015. PMID: 26405764 Free PMC article.
References
-
- Greene WC, Peterlin B. Charting HIV's remarkable voyage through the cell. Basic science as a passport to future therapy. Nat Med. 2002;8:673–680. - PubMed
-
- Chun TW, Engel D, Mizell SB, Hallahan C, Fischette M, et al. Effect of interleukin-2 on the pool of latently infected, resting CD4+ T cells in HIV-1-infected patients receiving highly active anti-retroviral therapy. Nat Med. 1999;5:651–655. - PubMed
-
- Gulick RM, Mellors JW, Havlir D, Eron JJ, Gonzalez C, et al. Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. N Engl J Med. 1997;337:734–739. - PubMed
-
- Hammer SM, Squires KE, Hughes MD, Grimes JM, Demeter LM, et al. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. AIDS Clinical Trials Group 320 Study Team. N Engl J Med. 1997;337:725–733. - PubMed
-
- Palella FJ, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–860. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

