Tolerance and safety evaluation in a large cohort of healthy infants fed an innovative prebiotic formula: a randomized controlled trial
- PMID: 22140499
- PMCID: PMC3227609
- DOI: 10.1371/journal.pone.0028010
Tolerance and safety evaluation in a large cohort of healthy infants fed an innovative prebiotic formula: a randomized controlled trial
Abstract
Background: the addition of oligosaccharides to infant formula has been shown to mimic some of the beneficial effects of human milk. The aim of the study was to assess the tolerance and safety of a formula containing an innovative mixture of oligosaccharides in early infancy.
Methodology/principal findings: this study was performed as a multi-center, randomized, double-blind, placebo-controlled trial including healthy term infants. Infants were recruited before the age of 8 weeks, either having started with formula feeding or being fully breast-fed (breastfeeding group). Formula-fed infants were randomized to feeding with a regular formula containing a mixture of neutral oligosaccharides and pectin-derived acidic oligosaccharides (prebiotic formula group) or regular formula without oligosaccharides (control formula group). Growth, tolerance and adverse events were assessed at 8, 16, 24 and 52 weeks of age. The prebiotic and control groups showed similar mean weight, length and head circumference, skin fold thicknesses, arm circumference gains and stool frequency at each study point. As far as the anthropometric parameters are concerned, the prebiotic group and the control group did not attain the values shown by the breastfeeding group at any study point. The skin fold thicknesses assessed in the breastfeeding group at 8 weeks were strikingly larger than those in formula fed infants, whereas at 52 weeks were strikingly smaller. The stool consistency in the prebiotic group was softer than in the control group at 8, 16 and 24 weeks (p<0.001) and closer to that of the breastfeeding group. There was no difference in the incidence of adverse events between the two formula groups.
Conclusions: our findings demonstrate the tolerability and the long term safety of a formula containing an innovative mixture of oligosaccharides in a large cohort of healthy infants.
Trial registration: drks-neu.uniklinik-freiburg.de DRKS 00000201.
Conflict of interest statement
Figures

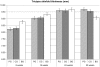

References
-
- Boehm G, Moro G. Structural and functional aspects of prebiotics used in infant nutrition. J146;Nutr. 2008;138:1818S–1828S. - PubMed
-
- Boehm G, Stahl B, Jelinek J, Knol J, Miniello V, et al. Prebiotic carbohydrates in human milk and formulas. Acta Paediatr. 2005;Suppl. 94:18–21. - PubMed
-
- Harmsen HJ, Wildeboer-Veloo AC, Raangs GC, Wagendorp AA, Klijn N, et al. Analysis of intestinal flora development in breast-fed and formula-fed infants by using molecular identification and detection methods. J146;Pediatr Gastroenterol Nutr. 2000;30:61–67. - PubMed
-
- Field CJ. The immunological components of human milk and their effect on immune development in infants. J146;Nutr. 2005;135:1–4. - PubMed
-
- Boehm G, Stahl B. Oligosaccharides. In: Mattila-Sandholm T, editor. Functional dairy products. Cambridge: Woodhead Publ; 2003. pp. 203–243.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

