Congenital cataract causing mutants of αA-crystallin/sHSP form aggregates and aggresomes degraded through ubiquitin-proteasome pathway
- PMID: 22140512
- PMCID: PMC3226672
- DOI: 10.1371/journal.pone.0028085
Congenital cataract causing mutants of αA-crystallin/sHSP form aggregates and aggresomes degraded through ubiquitin-proteasome pathway
Abstract
Background: Mutations of human αA-crystallin cause congenital cataract by protein aggregation. How mutations of αA-crystallin cause disease pathogenesis through protein aggregation is not well understood. To better understand the cellular events leading to protein aggregation, we transfected cataract causing mutants, R12C, R21L, R21W, R49C, R54C, R116C and R116H, of human αA-crystallin in HeLa cells and examined the formation of intracellular protein aggregates and aggresomes by confocal microscopy.
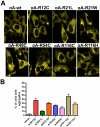
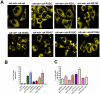
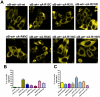
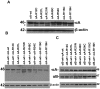
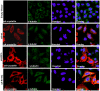
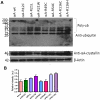
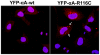
Methodology/principal findings: YFP-tagged human αA-wild-type (αA-wt) was sub-cloned and the mutants were generated by site-directed mutagenesis. The αA-wt and the mutants were individually transfected or co-transfected with CFP-tagged αA-wt or αB-wild-type (αB-wt) in HeLa cells. Overexpression of these mutants forms multiple small dispersed cytoplasmic aggregates as well as aggresomes. Co-expression of αB-wt with these mutants significantly inhibited protein aggregates where as co-expression with αA-wt enhanced protein aggregates which seems to be due to co-aggregation of the mutants with αA-wt. Aggresomes were validated by double immunofluorescence by co-localization of γ-tubulin, a centrosome marker protein with αA-crystallin. Furthermore, increased ubiquitination was detected in R21W, R116C and R116H as assessed by western blot analyses. Immunostaining with an ubiquitin antibody revealed that ubiquitin inclusions in the perinuclear regions were evident only in R116C transfected cells. Pulse chase assay, after cycloheximide treatment, suggested that R116C degraded faster than the wild-type control.
Conclusions/significance: Mutants of αA-crystallin form aggregates and aggresomes. Co-expression of αA-wt with the mutants increased aggregates and co-expression of αB-wt with the mutants significantly decreased the aggregates. The mutant, R116C protein degraded faster than wild-type control and increased ubiquitination was evident in R116C expressing cells.
Conflict of interest statement
Figures

Similar articles
-
Comparison of effect of gamma ray irradiation on wild-type and N-terminal mutants of αA-crystallin.Mol Vis. 2014 Jul 7;20:1002-16. eCollection 2014. Mol Vis. 2014. PMID: 25018622 Free PMC article.
-
Quaternary structural parameters of the congenital cataract causing mutants of αA-crystallin.Mol Cell Biochem. 2012 Mar;362(1-2):93-102. doi: 10.1007/s11010-011-1131-8. Epub 2011 Nov 2. Mol Cell Biochem. 2012. PMID: 22045060 Free PMC article.
-
Mutations in human αA-crystallin/sHSP affect subunit exchange interaction with αB-crystallin.PLoS One. 2012;7(2):e31421. doi: 10.1371/journal.pone.0031421. Epub 2012 Feb 8. PLoS One. 2012. PMID: 22347476 Free PMC article.
-
Regulation of αA- and αB-crystallins via phosphorylation in cellular homeostasis.Cell Mol Life Sci. 2015 Nov;72(21):4127-37. doi: 10.1007/s00018-015-1996-x. Epub 2015 Jul 26. Cell Mol Life Sci. 2015. PMID: 26210153 Free PMC article. Review.
-
Contrast Functions of αA- and αB-Crystallins in Cancer Development.Curr Mol Med. 2017;16(10):914-922. doi: 10.2174/1566524016666161223110508. Curr Mol Med. 2017. PMID: 28017134 Review.
Cited by
-
The Role of Small Heat Shock Proteins in Protein Misfolding Associated Motoneuron Diseases.Int J Mol Sci. 2022 Oct 4;23(19):11759. doi: 10.3390/ijms231911759. Int J Mol Sci. 2022. PMID: 36233058 Free PMC article. Review.
-
BetaB2-crystallin mutations associated with cataract and glaucoma leads to mitochondrial alterations in lens epithelial cells and retinal neurons.Exp Eye Res. 2017 Feb;155:85-90. doi: 10.1016/j.exer.2017.01.005. Epub 2017 Jan 26. Exp Eye Res. 2017. PMID: 28131617 Free PMC article.
-
Comparison of effect of gamma ray irradiation on wild-type and N-terminal mutants of αA-crystallin.Mol Vis. 2014 Jul 7;20:1002-16. eCollection 2014. Mol Vis. 2014. PMID: 25018622 Free PMC article.
-
Expression Profiling of DNA Methylation and Transcriptional Repression Associated Genes in Lens Epithelium Cells of Age-Related Cataract.Cell Mol Neurobiol. 2017 Apr;37(3):537-543. doi: 10.1007/s10571-016-0393-9. Epub 2016 Jun 15. Cell Mol Neurobiol. 2017. PMID: 27306760 Free PMC article.
-
A novel mutation in the CRYAA gene associated with congenital cataract and microphthalmia in a Chinese family.BMC Med Genet. 2018 Oct 19;19(1):190. doi: 10.1186/s12881-018-0695-5. BMC Med Genet. 2018. PMID: 30340470 Free PMC article.
References
-
- Reddy MA, Francis JP, Berry V, Bhattacharya S, Moore AT. Molecular genetic basis of inherited cataract and associated phenotypes. Sur Opthalmol. 2004;49:300–315. - PubMed
-
- De Jong WW, Terwindt EC, Bloemendal H. The amino acid sequence of the A chain of human alpha-crystallin. FEBS Lett. 1975;58:310–313. - PubMed
-
- Hansen L, Yao W, Eiberg H, Kjaer KW, Baggesen K, et al. Genetic heterogeneity in microcornea-cataract: five novel mutations in CRYAA, CRYGD and GJA8. Invest Opthalmol Vis Sci. 2007;48:3937–3944. - PubMed
-
- Xia C, Liu H, Chang B, Cheung D, Wang M, et al. Arginine 54 and Tyrosine 118 Residues of αA-Crystallin are Crucial for Lens Formation and Transparency. Invest Opthalmol Vis Sci. 2006;47:3004–3010. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

