Endocannabinoid regulation of acute and protracted nicotine withdrawal: effect of FAAH inhibition
- PMID: 22140525
- PMCID: PMC3227620
- DOI: 10.1371/journal.pone.0028142
Endocannabinoid regulation of acute and protracted nicotine withdrawal: effect of FAAH inhibition
Abstract
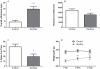
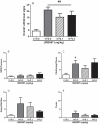
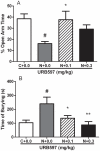
Evidence shows that the endocannabinoid system modulates the addictive properties of nicotine. In the present study, we hypothesized that spontaneous withdrawal resulting from removal of chronically implanted transdermal nicotine patches is regulated by the endocannabinoid system. A 7-day nicotine dependence procedure (5.2 mg/rat/day) elicited occurrence of reliable nicotine abstinence symptoms in Wistar rats. Somatic and affective withdrawal signs were observed at 16 and 34 hours following removal of nicotine patches, respectively. Further behavioral manifestations including decrease in locomotor activity and increased weight gain also occurred during withdrawal. Expression of spontaneous nicotine withdrawal was accompanied by fluctuation in levels of the endocannabinoid anandamide (AEA) in several brain structures including the amygdala, the hippocampus, the hypothalamus and the prefrontal cortex. Conversely, levels of 2-arachidonoyl-sn-glycerol were not significantly altered. Pharmacological inhibition of fatty acid amide hydrolase (FAAH), the enzyme responsible for the intracellular degradation of AEA, by URB597 (0.1 and 0.3 mg/kg, i.p.), reduced withdrawal-induced anxiety as assessed by the elevated plus maze test and the shock-probe defensive burying paradigm, but did not prevent the occurrence of somatic signs. Together, the results indicate that pharmacological strategies aimed at enhancing endocannabinoid signaling may offer therapeutic advantages to treat the negative affective state produced by nicotine withdrawal, which is critical for the maintenance of tobacco use.
Conflict of interest statement
Figures
References
-
- WHO. The World Health Report 2002: Reducing Risk, Promoting Healthy Life. 2002. Geneve. - PubMed
-
- Risner ME, Goldberg SR. A comparison of nicotine and cocaine self-administration in the dog: Fixed-ratio and progressive ratio schedules of intravenous drug infusion. J Pharmavol Exp Ther. 1983;224:319–326. - PubMed
-
- Pomerleau OF. Nicotine as a psychoactive drug: anxiety and pain reduction. Psychopharmacol Bull. 1986;22:865–869. - PubMed
-
- Watkins SS, Stinus L, Koob GF, Markou A. Reward and somatic changes during precipitated nicotine withdrawal in rats: centrally and peripherally mediated effects. J Pharmacol Exp Ther. 2000;292:1053–1064. - PubMed
-
- Kenny PJ, Markou A. Neurobiology of the nicotine withdrawal syndrome. Pharmacol Biochem Behav. 2001;70:531–549. - PubMed

