Convergent sets of data from in vivo and in vitro methods point to an active role of Hsp60 in chronic obstructive pulmonary disease pathogenesis
- PMID: 22140545
- PMCID: PMC3225395
- DOI: 10.1371/journal.pone.0028200
Convergent sets of data from in vivo and in vitro methods point to an active role of Hsp60 in chronic obstructive pulmonary disease pathogenesis
Abstract
Background: It is increasingly clear that some heat shock proteins (Hsps) play a role in inflammation. Here, we report results showing participation of Hsp60 in the pathogenesis of chronic obstructive pulmonary diseases (COPD), as indicated by data from both in vivo and in vitro analyses.
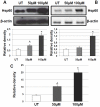
Methods and results: Bronchial biopsies from patients with stable COPD, smoker controls with normal lung function, and non-smoker controls were studied. We quantified by immunohistochemistry levels of Hsp10, Hsp27, Hsp40, Hsp60, Hsp70, Hsp90, and HSF-1, along with levels of inflammatory markers. Hsp10, Hsp40, and Hsp60 were increased during progression of disease. We found also a positive correlation between the number of neutrophils and Hsp60 levels. Double-immunostaining showed that Hsp60-positive neutrophils were significantly increased in COPD patients. We then investigated in vitro the effect on Hsp60 expression in bronchial epithelial cells (16HBE) caused by oxidative stress, a hallmark of COPD mucosa, which we induced with H₂O₂. This stressor determined increased levels of Hsp60 through a gene up-regulation mechanism involving NFkB-p65. Release of Hsp60 in the extracellular medium by the bronchial epithelial cells was also increased after H₂O₂ treatment in the absence of cell death.
Conclusions: This is the first report clearly pointing to participation of Hsps, particularly Hsp60, in COPD pathogenesis. Hsp60 induction by NFkB-p65 and its release by epithelial cells after oxidative stress can have a role in maintaining inflammation, e.g., by stimulating neutrophils activity. The data open new scenarios that might help in designing efficacious anti-inflammatory therapies centered on Hsp60 and applicable to COPD.
Conflict of interest statement
Figures




Similar articles
-
Hsp60 and Hsp10 down-regulation predicts bronchial epithelial carcinogenesis in smokers with chronic obstructive pulmonary disease.Cancer. 2006 Nov 15;107(10):2417-24. doi: 10.1002/cncr.22265. Cancer. 2006. PMID: 17048249
-
Hsp10 nuclear localization and changes in lung cells response to cigarette smoke suggest novel roles for this chaperonin.Open Biol. 2014 Oct;4(10):140125. doi: 10.1098/rsob.140125. Open Biol. 2014. PMID: 25355063 Free PMC article.
-
Beta defensin-2 is reduced in central but not in distal airways of smoker COPD patients.PLoS One. 2012;7(3):e33601. doi: 10.1371/journal.pone.0033601. Epub 2012 Mar 16. PLoS One. 2012. PMID: 22438960 Free PMC article.
-
Cellular and molecular mechanisms in chronic obstructive pulmonary disease: an overview.Clin Exp Allergy. 2004 Aug;34(8):1156-67. doi: 10.1111/j.1365-2222.2004.02030.x. Clin Exp Allergy. 2004. PMID: 15298554 Review.
-
[Diagnostic use of bronchoscopy and bronchial biopsies in chronic obstructive pulmonary disease].Nihon Rinsho. 2003 Dec;61(12):2158-62. Nihon Rinsho. 2003. PMID: 14674326 Review. Japanese.
Cited by
-
Response to: Hsp27 and Hsp70 in chronic obstructive pulmonary disease.Cell Stress Chaperones. 2015 Sep;20(5):725-6. doi: 10.1007/s12192-015-0620-1. Epub 2015 Jul 24. Cell Stress Chaperones. 2015. PMID: 26205190 Free PMC article. No abstract available.
-
Elevated blood Hsp60, its structural similarities and cross-reactivity with thyroid molecules, and its presence on the plasma membrane of oncocytes point to the chaperonin as an immunopathogenic factor in Hashimoto's thyroiditis.Cell Stress Chaperones. 2014 May;19(3):343-53. doi: 10.1007/s12192-013-0460-9. Epub 2013 Sep 22. Cell Stress Chaperones. 2014. PMID: 24057177 Free PMC article.
-
Exosome regulation of immune response mechanism: Pros and cons in immunotherapy.Bioact Mater. 2023 Oct 4;32:124-146. doi: 10.1016/j.bioactmat.2023.09.018. eCollection 2024 Feb. Bioact Mater. 2023. PMID: 37927901 Free PMC article.
-
Innate immunity but not NLRP3 inflammasome activation correlates with severity of stable COPD.Thorax. 2014 Jun;69(6):516-24. doi: 10.1136/thoraxjnl-2012-203062. Epub 2014 Jan 15. Thorax. 2014. PMID: 24430176 Free PMC article.
-
Inflammatory stress and sarcomagenesis: a vicious interplay.Cell Stress Chaperones. 2014 Jan;19(1):1-13. doi: 10.1007/s12192-013-0449-4. Epub 2013 Aug 27. Cell Stress Chaperones. 2014. PMID: 24046208 Free PMC article. Review.
References
-
- National Institutes of Health, National Heart, Lung and Blood Institute. Global Initiative for Chronic Obstructive Lung Disease (GOLD): global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease. NHLBI/WHO workshop report. NIH Publication No 2701A. Available: http://www.goldcopd.com/. Accessed 2011 March 28)
-
- Di Stefano A, Caramori G, Ricciardolo FL, Capelli A, Adcock IM, et al. Cellular and molecular mechanisms in chronic obstructive pulmonary disease: an overview. Clin Exp Allergy. 2004;34:1156–1167. - PubMed
-
- Yoo CG, Lee S, Lee CT, Kim YW, Han SK, et al. Anti-inflammatory effect of heat shock protein induction is related to stabilization of I kappa B alpha through preventing I kappa B kinase activation in respiratory epithelial cells. J Immunol. 2000;164:5416–5423. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

