MUC1-C oncoprotein regulates glycolysis and pyruvate kinase M2 activity in cancer cells
- PMID: 22140559
- PMCID: PMC3225393
- DOI: 10.1371/journal.pone.0028234
MUC1-C oncoprotein regulates glycolysis and pyruvate kinase M2 activity in cancer cells
Abstract
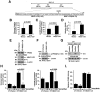
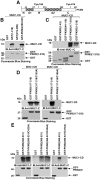
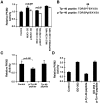
Aerobic glycolysis in cancer cells is regulated by multiple effectors that include Akt and pyruvate kinase M2 (PKM2). Mucin 1 (MUC1) is a heterodimeric glycoprotein that is aberrantly overexpressed by human breast and other carcinomas. Here we show that transformation of rat fibroblasts by the oncogenic MUC1-C subunit is associated with Akt-mediated increases in glucose uptake and lactate production, consistent with the stimulation of glycolysis. The results also demonstrate that the MUC1-C cytoplasmic domain binds directly to PKM2 at the B- and C-domains. Interaction between the MUC1-C cytoplasmic domain Cys-3 and the PKM2 C-domain Cys-474 was found to stimulate PKM2 activity. Conversely, epidermal growth factor receptor (EGFR)-mediated phosphorylation of the MUC1-C cytoplasmic domain on Tyr-46 conferred binding to PKM2 Lys-433 and inhibited PKM2 activity. In human breast cancer cells, silencing MUC1-C was associated with decreases in glucose uptake and lactate production, confirming involvement of MUC1-C in the regulation of glycolysis. In addition, EGFR-mediated phosphorylation of MUC1-C in breast cancer cells was associated with decreases in PKM2 activity. These findings indicate that the MUC1-C subunit regulates glycolysis and that this response is conferred in part by PKM2. Thus, the overexpression of MUC1-C oncoprotein in diverse human carcinomas could be of importance to the Warburg effect of aerobic glycolysis.
Conflict of interest statement
Figures






Similar articles
-
Beta-elemene inhibits breast cancer metastasis through blocking pyruvate kinase M2 dimerization and nuclear translocation.J Cell Mol Med. 2019 Oct;23(10):6846-6858. doi: 10.1111/jcmm.14568. Epub 2019 Jul 25. J Cell Mol Med. 2019. PMID: 31343107 Free PMC article.
-
Mammalian target of rapamycin up-regulation of pyruvate kinase isoenzyme type M2 is critical for aerobic glycolysis and tumor growth.Proc Natl Acad Sci U S A. 2011 Mar 8;108(10):4129-34. doi: 10.1073/pnas.1014769108. Epub 2011 Feb 15. Proc Natl Acad Sci U S A. 2011. PMID: 21325052 Free PMC article.
-
p,p'-Dichlorodiphenyltrichloroethane promotes aerobic glycolysis via reactive oxygen species-mediated extracellular signal-regulated kinase/M2 isoform of pyruvate kinase (PKM2) signaling in colorectal cancer cells.Environ Toxicol. 2020 Mar;35(3):333-345. doi: 10.1002/tox.22869. Epub 2019 Nov 13. Environ Toxicol. 2020. PMID: 31724279
-
MUC1-C oncoprotein as a target in breast cancer: activation of signaling pathways and therapeutic approaches.Oncogene. 2013 Feb 28;32(9):1073-81. doi: 10.1038/onc.2012.158. Epub 2012 May 14. Oncogene. 2013. PMID: 22580612 Free PMC article. Review.
-
Pyruvate kinase M2: multiple faces for conferring benefits on cancer cells.Clin Cancer Res. 2012 Oct 15;18(20):5554-61. doi: 10.1158/1078-0432.CCR-12-0859. Clin Cancer Res. 2012. PMID: 23071357 Review.
Cited by
-
MUC1-C integrates aerobic glycolysis with suppression of oxidative phosphorylation in triple-negative breast cancer stem cells.iScience. 2023 Oct 11;26(11):108168. doi: 10.1016/j.isci.2023.108168. eCollection 2023 Nov 17. iScience. 2023. PMID: 37915591 Free PMC article.
-
Mucin-1: a promising pan-cancer therapeutic target.NPJ Precis Oncol. 2025 Jul 2;9(1):218. doi: 10.1038/s41698-025-01016-2. NPJ Precis Oncol. 2025. PMID: 40604087 Free PMC article. Review.
-
Influence of the MUC1 Cell Surface Mucin on Gastric Mucosal Gene Expression Profiles in Response to Helicobacter pylori Infection in Mice.Front Cell Infect Microbiol. 2020 Jul 24;10:343. doi: 10.3389/fcimb.2020.00343. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32793510 Free PMC article.
-
Mucins reprogram stemness, metabolism and promote chemoresistance during cancer progression.Cancer Metastasis Rev. 2021 Jun;40(2):575-588. doi: 10.1007/s10555-021-09959-1. Epub 2021 Apr 4. Cancer Metastasis Rev. 2021. PMID: 33813658 Free PMC article. Review.
-
Mucin1 and Mucin16: Therapeutic Targets for Cancer Therapy.Pharmaceuticals (Basel). 2021 Oct 17;14(10):1053. doi: 10.3390/ph14101053. Pharmaceuticals (Basel). 2021. PMID: 34681277 Free PMC article. Review.
References
-
- Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. - PubMed
-
- Majumder PK, Febbo PG, Bikoff R, Berger R, Xue Q, et al. mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nat Med. 2004;10:594–601. - PubMed
-
- Altenberg B, Greulich KO. Genes of glycolysis are ubiquitously overexpressed in 24 cancer classes. Genomics. 2004;84:1014–1020. - PubMed
-
- Mazurek S, Boschek CB, Hugo F, Eigenbrodt E. Pyruvate kinase type M2 and its role in tumor growth and spreading. Semin Cancer Biol. 2005;15:300–308. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

